Evidence for behavioral benefits of early dietary supplementation with CoEnzymeQ10 in a slowly progressing mouse model of Huntington's disease
- PMID: 22044764
- PMCID: PMC3278578
- DOI: 10.1016/j.mcn.2011.10.007
Evidence for behavioral benefits of early dietary supplementation with CoEnzymeQ10 in a slowly progressing mouse model of Huntington's disease
Abstract
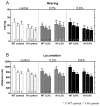
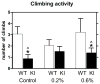
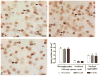
Controversies surround the usefulness of Coenzyme Q10 (CoQ10) in Huntington's disease (HD), an autosomal dominant, fatal, neurodegenerative disease with no cure or disease modifying treatment. CoQ10, an endogenous substrate for electron transport and an anti-oxidant, has been shown in some but not all studies to improve symptoms and survival in mouse models of HD. Previous studies have been conducted in fast-progressing models that better mimic the juvenile forms of HD than the much more common middle-age onset form, possibly accounting for mixed results. Establishing the usefulness of CoQ10 to alter HD disease course in a model that better recapitulates the progressive features of the human disorder is important because clinical trials of CoQ10, which is safe and well tolerated, are being planned in patients. The CAG140 knock-in (KI) mouse model of HD in which an expanded (approximately 120) CAG repeat is inserted in the mouse gene provides a model of the mutation in the proper genomic and protein context. These mice display progressive motor, cognitive and emotional anomalies, transcriptional disturbances and late striatal degeneration. Homozygote mutant CAG140 KI mice and wild-type littermates were fed CoQ10 (0.2%, 0.6%) in chow, and behavioral and pathological markers of disease were examined. CoQ10 improved early behavioral deficits and normalized some transcriptional deficits without altering huntingtin aggregates in striatum. The lower dose (0.2%) was more beneficial than 0.6%. Similar to previous studies, this low dose also induced deleterious effects in open field and rotarod in WT mice, however these effects are of unclear clinical significance in view of the excellent safety profile of CoQ10 in humans. These data confirm that CoQ10 may be beneficial in HD but suggest that maximum benefit may be observed when treatment is begun at early stages of the disease and that dosage may be critical.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest.
Figures


Similar articles
-
Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington's disease.Mol Neurodegener. 2012 Apr 4;7:12. doi: 10.1186/1750-1326-7-12. Mol Neurodegener. 2012. PMID: 22475209 Free PMC article.
-
Combination therapy using minocycline and coenzyme Q10 in R6/2 transgenic Huntington's disease mice.Biochim Biophys Acta. 2006 Mar;1762(3):373-80. doi: 10.1016/j.bbadis.2005.11.002. Epub 2005 Dec 5. Biochim Biophys Acta. 2006. PMID: 16364609
-
Dose ranging and efficacy study of high-dose coenzyme Q10 formulations in Huntington's disease mice.Biochim Biophys Acta. 2006 Jun;1762(6):616-26. doi: 10.1016/j.bbadis.2006.03.004. Epub 2006 Apr 17. Biochim Biophys Acta. 2006. PMID: 16647250
-
Coenzyme Q10 as a possible treatment for neurodegenerative diseases.Free Radic Res. 2002 Apr;36(4):455-60. doi: 10.1080/10715760290021315. Free Radic Res. 2002. PMID: 12069110 Review.
-
Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases.Biofactors. 1999;9(2-4):261-6. doi: 10.1002/biof.5520090222. Biofactors. 1999. PMID: 10416039 Review.
Cited by
-
Moderate Intensity Exercise in Pre-manifest Huntington's Disease: Results of a 6 months Trial.SVOA Neurol. 2021;2(1):6-36. Epub 2021 Feb 3. SVOA Neurol. 2021. PMID: 35128541 Free PMC article.
-
Euglycemic agent-mediated hypothalamic transcriptomic manipulation in the N171-82Q model of Huntington disease is related to their physiological efficacy.J Biol Chem. 2012 Sep 14;287(38):31766-82. doi: 10.1074/jbc.M112.387316. Epub 2012 Jul 20. J Biol Chem. 2012. PMID: 22822065 Free PMC article.
-
Use of a force-sensing automated open field apparatus in a longitudinal study of multiple behavioral deficits in CAG140 Huntington's disease model mice.Behav Brain Res. 2015 Nov 1;294:7-16. doi: 10.1016/j.bbr.2015.07.036. Epub 2015 Jul 22. Behav Brain Res. 2015. PMID: 26210937 Free PMC article.
-
Coenzyme Q10 effects in neurological diseases.Physiol Res. 2021 Dec 30;70(Suppl4):S683-S714. doi: 10.33549/physiolres.934712. Physiol Res. 2021. PMID: 35199552 Free PMC article. Review.
-
Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington's disease.Mol Neurodegener. 2012 Apr 4;7:12. doi: 10.1186/1750-1326-7-12. Mol Neurodegener. 2012. PMID: 22475209 Free PMC article.
References
-
- Andrich J, Saft C, Gerlach M, Schneider B, Arz A, Kuhn W, Muller T. Coenzyme Q10 serum levels in Huntington’s disease. J Neural Transm Suppl. 2004:111–116. - PubMed
-
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. - PubMed
-
- Aylward EH. Change in MRI striatal volumes as a biomarker in preclinical Huntington’s disease. Brain Res Bull. 2007;72:152–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

