Rbfox proteins regulate alternative splicing of neuronal sodium channel SCN8A
- PMID: 22044765
- PMCID: PMC3278527
- DOI: 10.1016/j.mcn.2011.10.005
Rbfox proteins regulate alternative splicing of neuronal sodium channel SCN8A
Abstract
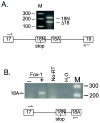
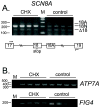
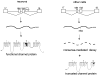
The SCN8A gene encodes the voltage-gated sodium channel Na(v)1.6, a major channel in neurons of the CNS and PNS. SCN8A contains two alternative exons,18N and 18A, that exhibit tissue specific splicing. In brain, the major SCN8A transcript contains exon 18A and encodes the full-length sodium channel. In other tissues, the major transcript contains exon 18N and encodes a truncated protein, due to the presence of an in-frame stop codon. Selection of exon 18A is therefore essential for generation of a functional channel protein, but the proteins involved in this selection have not been identified. Using a 2.6 kb Scn8a minigene containing exons 18N and 18A, we demonstrate that co-transfection with Fox-1 or Fox-2 initiates inclusion of exon 18A. This effect is dependent on the consensus Fox binding site located 28 bp downstream of exon 18A. We examined the alternative splicing of human SCN8A and found that the postnatal switch to exon 18A is completed later than 10 months of age. In purified cell populations, transcripts containing exon 18A predominate in neurons but are not present in oligodendrocytes or astrocytes. Transcripts containing exon 18N appear to be degraded by nonsense-mediated decay in HEK cells. Our data indicate that RBFOX proteins contribute to the cell-specific expression of Na(v)1.6 channels in mature neurons.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- ASSP splice predictor. http://www.es.embnet.org/~mwang/assp.html.
-
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
-
- Barres BA, Koroshetz WJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the type-1 astrocyte. Neuron. 1990;5:527–544. - PubMed
-
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

