Correction of murine Bernard-Soulier syndrome by lentivirus-mediated gene therapy
- PMID: 22044935
- PMCID: PMC3293608
- DOI: 10.1038/mt.2011.231
Correction of murine Bernard-Soulier syndrome by lentivirus-mediated gene therapy
Abstract
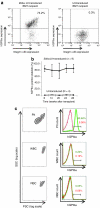
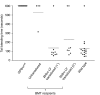
Bernard-Soulier syndrome (BSS) is an inherited bleeding disorder caused by a defect in the platelet glycoprotein (GP) Ib-IX-V complex. The main treatment for BSS is platelet transfusion but it is often limited to severe bleeding episodes or surgical interventions due to the risk of alloimmunization. We have previously reported successful expression of human GPIbα (hGPIbα) in human megakaryocytes using a lentiviral vector (LV) encoding human GP1BA under control of the platelet-specific integrin αIIb promoter (2bIbα). In this study, we examined the efficacy of this strategy for the gene therapy of BSS using GPIbα(null) as a murine model of BSS. GPIbα(null) hematopoietic stem cells (HSC) transduced with 2bIbα LV were transplanted into lethally irradiated GPIbα(null) littermates. Therapeutic levels of hGPIbα expression were achieved that corrected the tail bleeding time and improved the macrothrombocytopenia. Sequential bone marrow (BM) transplants showed sustained expression of hGPIbα with similar phenotypic correction. Antibody response to hGPIbα was documented in 1 of 17 total recipient mice but was tolerated without any further treatment. These results demonstrate that lentivirus-mediated gene transfer can provide sustained phenotypic correction of murine BSS, indicating that this approach may be a promising strategy for gene therapy of BSS patients.
Figures




References
-
- Bernard J., and, Soulier JP. [Not Available] Sem Hop. 1948;24 Spec. No.:3217–3223.
-
- Nurden P., and, Nurden AT. Congenital disorders associated with platelet dysfunctions. Thromb Haemost. 2008;99:253–263. - PubMed
-
- Clemetson KJ, McGregor JL, James E, Dechavanne M., and, Lüscher EF. Characterization of the platelet membrane glycoprotein abnormalities in Bernard-Soulier syndrome and comparison with normal by surface-labeling techniques and high-resolution two-dimensional gel electrophoresis. J Clin Invest. 1982;70:304–311. - PMC - PubMed
-
- López JA, Andrews RK, Afshar-Kharghan V., and, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91:4397–4418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

