Consumption of high ω-3 fatty acid diet suppressed prostate tumorigenesis in C3(1) Tag mice
- PMID: 22045025
- PMCID: PMC3276330
- DOI: 10.1093/carcin/bgr238
Consumption of high ω-3 fatty acid diet suppressed prostate tumorigenesis in C3(1) Tag mice
Abstract
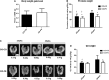
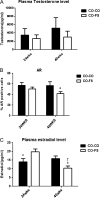
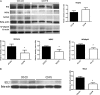
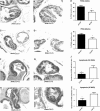
Prostate cancer incidence and mortality are high in the Western world and high ω-6/ω-3 PUFA in the Western diet may be a contributing factor. We investigated whether changing from a diet that approximates ω-6 fat content of the Western diet to a high ω-3 fat diet at adulthood might reduce prostate cancer risk. Female SV 129 mice that had consumed a high ω-6 diet containing corn oil for 2 weeks were bred with homozygous C3(1)Tag transgenic male mice. All male offspring were weaned to the corn oil diet (CO) until postpuberty when half of the male offspring were transferred to a high ω-3 diet containing canola oil and fish oil concentrate (FS). High ω-3 diet increased ω-3 and decreased ω-6 fat content of mice tissues. Average weights of prostate and genitourinary bloc were significantly lower in mice consuming high ω-3 diet at adulthood (CO-FS) than mice fed a lifetime high ω-6 diet (CO-CO). There was slower progression of tumorigenesis in dorsalateral prostate of CO-FS than in CO-CO mice. CO-FS mice had slightly lower plasma testosterone level at 24 and 40 weeks, significantly lower estradiol level at 40 weeks and significantly less expressed androgen receptor (AR) in the dorsalateral prostate at 40 weeks than CO-CO mice. Consumption of high ω-3 diet lowered the expression of genes expected to increase proliferation and decrease apoptosis in dorsalateral prostate. Our results suggest that consumption of high ω-3 diet slows down prostate tumorigenesis by lowering estradiol, testosterone and AR levels, promoting apoptosis and suppressing cell proliferation in C3(1)Tag mice.
Figures

References
-
- Jemal A, et al. Cancer statistics. CA Cancer J. Clin. 2010;60:277–300. - PubMed
-
- Jemal A, et al. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. - PubMed
-
- Platz E, et al. Prostate cancer. 3rd edn. In: Schottenfeld, D, Fraumeni, JF, Jr, editors. Cancer Epidemiology and Prevention. Oxford, UK,: Oxford University Press; 2006. 1128–1150[59]
-
- Astorg P. Dietary N-6 and N-3 polyunsaturated fatty acids and prostate cancer risk: a review of epidemiological and experimental evidence. Cancer Causes Control. 2004;15:367–386. - PubMed
-
- Pandalai PK, et al. The effects of omega-3 and omega-6 fatty acids on in vitro prostate cancer growth. Anticancer Res. 1996;16:815–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

