Palmitoylated TMX and calnexin target to the mitochondria-associated membrane
- PMID: 22045338
- PMCID: PMC3261551
- DOI: 10.1038/emboj.2011.384
Palmitoylated TMX and calnexin target to the mitochondria-associated membrane
Abstract
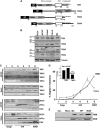
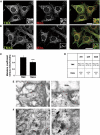
The mitochondria-associated membrane (MAM) is a domain of the endoplasmic reticulum (ER) that mediates the exchange of ions, lipids and metabolites between the ER and mitochondria. ER chaperones and oxidoreductases are critical components of the MAM. However, the localization motifs and mechanisms for most MAM proteins have remained elusive. Using two highly related ER oxidoreductases as a model system, we now show that palmitoylation enriches ER-localized proteins on the MAM. We demonstrate that palmitoylation of cysteine residue(s) adjacent to the membrane-spanning domain promotes MAM enrichment of the transmembrane thioredoxin family protein TMX. In addition to TMX, our results also show that calnexin shuttles between the rough ER and the MAM depending on its palmitoylation status. Mutation of the TMX and calnexin palmitoylation sites and chemical interference with palmitoylation disrupt their MAM enrichment. Since ER-localized heme oxygenase-1, but not cytosolic GRP75 require palmitoylation to reside on the MAM, our findings identify palmitoylation as key for MAM enrichment of ER membrane proteins.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A, Pinton P, Ripamonti M, Rizzuto R, Sitia R (2011) Ero1alpha regulates Ca2+ fluxes at the endoplasmic reticulum-mitochondria interface (MAM). Antioxid Redox Signal (in press) - PubMed
-
- Appenzeller-Herzog C, Ellgaard L (2008) The human PDI family: versatility packed into a single fold. Biochim Biophys Acta 1783: 535–548 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

