Ketamine induces motor neuron toxicity and alters neurogenic and proneural gene expression in zebrafish
- PMID: 22045596
- PMCID: PMC5474752
- DOI: 10.1002/jat.1751
Ketamine induces motor neuron toxicity and alters neurogenic and proneural gene expression in zebrafish
Abstract
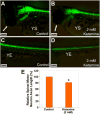
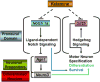
Ketamine, a noncompetitive antagonist of N-methyl-d-aspartate-type glutamate receptors, is a pediatric anesthetic that has been shown to be neurotoxic in rodents and nonhuman primates when administered during the brain growth spurt. Recently, the zebrafish has become an attractive model for toxicity assays, in part because the predictive capability of the zebrafish model, with respect to chemical effects, compares well with that from mammalian models. In the transgenic (hb9:GFP) embryos used in this study, green fluorescent protein (GFP) is expressed in the motor neurons, facilitating the visualization and analysis of motor neuron development in vivo. In order to determine whether ketamine induces motor neuron toxicity in zebrafish, embryos of these transgenic fish were treated with different concentrations of ketamine (0.5 and 2.0 mm). For ketamine exposures lasting up to 20 h, larvae showed no gross morphological abnormalities. Analysis of GFP-expressing motor neurons in the live embryos, however, revealed that 2.0 mm ketamine adversely affected motor neuron axon length and decreased cranial and motor neuron populations. Quantitative reverse transcriptase-polymerase chain reaction analysis demonstrated that ketamine down-regulated the motor neuron-inducing zinc finger transcription factor Gli2b and the proneural gene NeuroD even at 0.5 mm concentration, while up-regulating the expression of the proneural gene Neurogenin1 (Ngn1). Expression of the neurogenic gene, Notch1a, was suppressed, indicating that neuronal precursor generation from uncommitted cells was favored. These results suggest that ketamine is neurotoxic to motor neurons in zebrafish and possibly affects the differentiating/differentiatedneurons rather than neuronal progenitors. Published 2011. This article is a US Government work and is in the public domain in the USA.
Published 2011. This article is a US Government work and is in the public domain in the USA.
Figures



Similar articles
-
Acetyl L-carnitine protects motor neurons and Rohon-Beard sensory neurons against ketamine-induced neurotoxicity in zebrafish embryos.Neurotoxicol Teratol. 2013 Sep-Oct;39:69-76. doi: 10.1016/j.ntt.2013.07.005. Epub 2013 Jul 26. Neurotoxicol Teratol. 2013. PMID: 23896048 Free PMC article.
-
Effect of ketamine on gene expression in zebrafish embryos.J Appl Toxicol. 2021 Dec;41(12):2083-2089. doi: 10.1002/jat.4199. Epub 2021 May 17. J Appl Toxicol. 2021. PMID: 34002392
-
Ketamine attenuates cytochrome p450 aromatase gene expression and estradiol-17β levels in zebrafish early life stages.J Appl Toxicol. 2014 May;34(5):480-8. doi: 10.1002/jat.2888. Epub 2013 May 20. J Appl Toxicol. 2014. PMID: 23696345 Free PMC article.
-
Relationship between ketamine-induced developmental neurotoxicity and NMDA receptor-mediated calcium influx in neural stem cell-derived neurons.Neurotoxicology. 2017 May;60:254-259. doi: 10.1016/j.neuro.2016.04.015. Epub 2016 Apr 27. Neurotoxicology. 2017. PMID: 27132109 Review.
-
Dual effects of ketamine: neurotoxicity versus neuroprotection in anesthesia for the developing brain.J Neurosurg Anesthesiol. 2014 Apr;26(2):155-60. doi: 10.1097/ANA.0000000000000027. J Neurosurg Anesthesiol. 2014. PMID: 24275940 Review.
Cited by
-
Development of a primate model to evaluate the effects of ketamine and surgical stress on the neonatal brain.Exp Biol Med (Maywood). 2023 Apr;248(7):624-632. doi: 10.1177/15353702231168144. Epub 2023 May 19. Exp Biol Med (Maywood). 2023. PMID: 37208914 Free PMC article.
-
Cyclosporine exacerbates ketamine toxicity in zebrafish: Mechanistic studies on drug-drug interaction.J Appl Toxicol. 2017 Dec;37(12):1438-1447. doi: 10.1002/jat.3488. Epub 2017 Jun 1. J Appl Toxicol. 2017. PMID: 28569378 Free PMC article.
-
Ketamine-induced neurotoxicity in neurodevelopment: A synopsis of main pathways based on recent in vivo experimental findings.J Anaesthesiol Clin Pharmacol. 2021 Jan-Mar;37(1):37-42. doi: 10.4103/joacp.JOACP_415_19. Epub 2021 Apr 10. J Anaesthesiol Clin Pharmacol. 2021. PMID: 34103820 Free PMC article. Review.
-
Nifedipine toxicity is exacerbated by acetyl l-carnitine but alleviated by low-dose ketamine in zebrafish in vivo.J Appl Toxicol. 2020 Feb;40(2):257-269. doi: 10.1002/jat.3901. Epub 2019 Oct 9. J Appl Toxicol. 2020. PMID: 31599005 Free PMC article.
-
Ketamine exposure in early development impairs specification of the primary germ cell layers.Neurotoxicol Teratol. 2014 May-Jun;43:59-68. doi: 10.1016/j.ntt.2014.04.001. Epub 2014 Apr 16. Neurotoxicol Teratol. 2014. PMID: 24746641 Free PMC article.
References
-
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11(2):180–187. - PubMed
-
- Appel B, Eisen JS. Regulation of neuronal specification in the zebrafish spinal cord by Delta function. Development. 1998;125(3):371–380. - PubMed
-
- Appel B, Fritz A, Westerfield M, Grunwald DJ, Eisen JS, Riley BB. Delta-mediated specification of midline cell fates in zebrafish embryos. Curr Biol. 1999;9(5):247–256. - PubMed
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23(4):659–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

