Notch signaling is necessary to maintain quiescence in adult muscle stem cells
- PMID: 22045613
- PMCID: PMC3384696
- DOI: 10.1002/stem.773
Notch signaling is necessary to maintain quiescence in adult muscle stem cells
Abstract
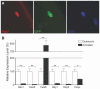
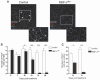
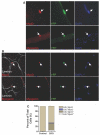
Satellite cells (SCs) are myogenic stem cells found in skeletal muscle that function to repair tissue damaged by injury or disease. SCs are quiescent at rest, although the signaling pathways required to maintain quiescence are unknown. Using a transgenic Notch reporter mouse and quantitative reverse-transcription polymerase chain reaction analysis of Notch target genes, we determined that Notch signaling is active in quiescent SCs. SC-specific deletion of recombining binding protein-Jκ (RBP-Jκ), a nuclear factor required for Notch signaling, resulted in the depletion of the SC pool and muscles that lacked any ability to regenerate in response to injury. SC depletion was not due to apoptosis. Rather, RBP-Jκ-deficient SCs spontaneously activate, fail to self-renew, and undergo terminal differentiation. Intriguingly, most of the cells differentiate without first dividing. They then fuse with adjacent myofibers, leading to the gradual disappearance of SCs from the muscle. These results demonstrate the requirement of Notch signaling for the maintenance of the quiescent state and for muscle stem cell homeostasis by the regulation of self-renewal and differentiation, processes that are all critical for normal postnatal myogenesis.
Copyright © 2011 AlphaMed Press.
Figures

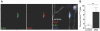

References
-
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. - PubMed
-
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: Genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. - PubMed
-
- Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

