Integrity of the network sarcoplasmic reticulum in skeletal muscle requires small ankyrin 1
- PMID: 22045734
- PMCID: PMC3215573
- DOI: 10.1242/jcs.085159
Integrity of the network sarcoplasmic reticulum in skeletal muscle requires small ankyrin 1
Abstract
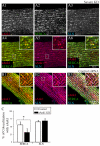
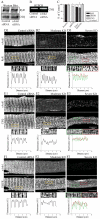
Small ankyrin 1 (sAnk1; Ank1.5) is a ~20 kDa protein of striated muscle that concentrates in the network compartment of the sarcoplasmic reticulum (nSR). We used siRNA targeted to sAnk1 to assess its role in organizing the sarcoplasmic reticulum (SR) of skeletal myofibers in vitro. siRNA reduced sAnk1 mRNA and protein levels and disrupted the organization of the remaining sAnk1. Sarcomeric proteins were unchanged, but two other proteins of the nSR, SERCA and sarcolipin, decreased significantly in amount and segregated into distinct structures containing sarcolipin and sAnk1, and SERCA, respectively. Exogenous sAnk1 restored SERCA to its normal distribution. Ryanodine receptors and calsequestrin in the junctional SR, and L-type Ca(2+) channels in the transverse tubules were not reduced, although their striated organization was mildly altered. Consistent with the loss of SERCA, uptake and release of Ca(2+) were significantly inhibited. Our results show that sAnk1 stabilizes the nSR and that its absence causes the nSR to fragment into distinct membrane compartments.
Figures







References
-
- Birkenmeier C. S., Sharp J. J., Gifford E. J., Deveau S. A., Barker J. E. (1998). An alternative first exon in the distal end of the erythroid ankyrin gene leads to production of a small isoform containing an NH2-terminal membrane anchor. Genomics 50, 79-88 - PubMed
-
- Borzok M. A., Catino D. H., Nicholson J. D., Kontrogianni-Konstantopoulos A., Bloch R. J. (2007). Mapping the binding site on small ankyrin 1 for obscurin. J. Biol. Chem. 282, 32384-32396 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

