Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1
- PMID: 22045807
- PMCID: PMC3247956
- DOI: 10.1074/jbc.M111.258053
Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1
Abstract
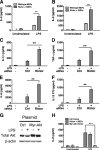
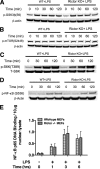
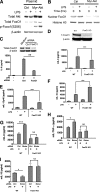
Activation of the PI3K pathway plays a pivotal role in regulating the inflammatory response. The loss of mTORC2 has been shown to abrogate the activation of Akt, a critical downstream component of PI3K signaling. However, the biological importance of mTORC2 in innate immunity is currently unknown. Here we demonstrate that rictor, a key component of mTORC2, plays a critical role in controlling the innate inflammatory response via its ability to regulate FoxO1. Upon LPS stimulation, both rictor-deficient mouse embryonic fibroblasts (MEFs) and rictor knockdown dendritic cells exhibited a hyperinflammatory phenotype. The hyperinflammatory phenotype was due to a defective Akt signaling axis, because both rictor-deficient MEFs and rictor knockdown dendritic cells exhibited attenuated Akt phosphorylation and kinase activity. Analysis of downstream Akt targets revealed that phosphorylation of FoxO1 was impaired in rictor-deficient cells, resulting in elevated nuclear FoxO1 levels and diminished nuclear export of FoxO1 upon LPS stimulation. Knockdown of FoxO1 attenuated the hyperinflammatory phenotype exhibited by rictor-deficient MEFs. Moreover, FoxO1 deletion in dendritic cells attenuated the capacity of LPS to induce inflammatory cytokine expression. These findings identify a novel signaling pathway by which mTORC2 regulates the TLR-mediated inflammatory response through its ability to regulate FoxO1.
Figures



References
-
- Cantley L. C. (2002) Science 296, 1655–1657 - PubMed
-
- Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Curr. Biol. 7, 261–269 - PubMed
-
- Stephens L., Anderson K., Stokoe D., Erdjument-Bromage H., Painter G. F., Holmes A. B., Gaffney P. R., Reese C. B., McCormick F., Tempst P., Coadwell J., Hawkins P. T. (1998) Science 279, 710–714 - PubMed
-
- Persad S., Attwell S., Gray V., Mawji N., Deng J. T., Leung D., Yan J., Sanghera J., Walsh M. P., Dedhar S. (2001) J. Biol. Chem. 276, 27462–27469 - PubMed
-
- Feng J., Park J., Cron P., Hess D., Hemmings B. A. (2004) J. Biol. Chem. 279, 41189–41196 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

