Degradation of p21Cip1 through anaphase-promoting complex/cyclosome and its activator Cdc20 (APC/CCdc20) ubiquitin ligase complex-mediated ubiquitylation is inhibited by cyclin-dependent kinase 2 in cardiomyocytes
- PMID: 22045811
- PMCID: PMC3243522
- DOI: 10.1074/jbc.M111.236711
Degradation of p21Cip1 through anaphase-promoting complex/cyclosome and its activator Cdc20 (APC/CCdc20) ubiquitin ligase complex-mediated ubiquitylation is inhibited by cyclin-dependent kinase 2 in cardiomyocytes
Abstract
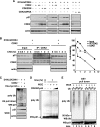
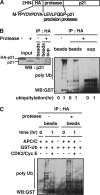
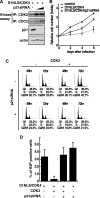
Cyclin-dependent kinase inhibitor p21Cip1 plays a crucial role in regulating cell cycle arrest and differentiation. It is known that p21Cip1 increases during terminal differentiation of cardiomyocytes, but its expression control and biological roles are not fully understood. Here, we show that the p21Cip1 protein is stabilized in cardiomyocytes after mitogenic stimulation, due to its increased CDK2 binding and inhibition of ubiquitylation. The APC/CCdc20 complex is shown to be an E3 ligase mediating ubiquitylation of p21Cip1 at the N terminus. CDK2, but not CDC2, suppressed the interaction of p21Cip1 with Cdc20, thereby leading to inhibition of anaphase-promoting complex/cyclosome and its activator Cdc20 (APC/CCdc20)-mediated p21Cip1 ubiquitylation. It was further demonstrated that p21Cip1 accumulation caused G2 arrest of cardiomyocytes that were forced to re-enter the cell cycle. Taken together, these data show that the stability of the p21Cip1 protein is actively regulated in terminally differentiated cardiomyocytes and plays a role in inhibiting their uncontrolled cell cycle progression. Our study provides a novel insight on the control of p21Cip1 by ubiquitin-mediated degradation and its implication in cell cycle arrest in terminal differentiation.
Figures


Similar articles
-
APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase.Mol Cell. 2007 Aug 3;27(3):462-73. doi: 10.1016/j.molcel.2007.06.013. Mol Cell. 2007. PMID: 17679094 Free PMC article.
-
Cdc20 protein contains a destruction-box but, unlike Clb2, its proteolysisis not acutely dependent on the activity of anaphase-promoting complex.Eur J Biochem. 2000 Jan;267(2):434-49. doi: 10.1046/j.1432-1327.2000.01014.x. Eur J Biochem. 2000. PMID: 10632713
-
The spindle checkpoint requires cyclin-dependent kinase activity.Genes Dev. 2003 Oct 15;17(20):2520-5. doi: 10.1101/gad.267603. Genes Dev. 2003. PMID: 14561775 Free PMC article.
-
Methods to measure ubiquitin-dependent proteolysis mediated by the anaphase-promoting complex.Methods. 2006 Jan;38(1):39-51. doi: 10.1016/j.ymeth.2005.07.005. Methods. 2006. PMID: 16343932 Review.
-
Cdc20: a WD40 activator for a cell cycle degradation machine.Mol Cell. 2007 Jul 6;27(1):3-16. doi: 10.1016/j.molcel.2007.06.009. Mol Cell. 2007. PMID: 17612486 Review.
Cited by
-
A novel, non-apoptotic role for Scythe/BAT3: a functional switch between the pro- and anti-proliferative roles of p21 during the cell cycle.PLoS One. 2012;7(6):e38085. doi: 10.1371/journal.pone.0038085. Epub 2012 Jun 27. PLoS One. 2012. PMID: 22761665 Free PMC article.
-
Global gene expression analysis combined with a genomics approach for the identification of signal transduction networks involved in postnatal mouse myocardial proliferation and development.Int J Mol Med. 2018 Jan;41(1):311-321. doi: 10.3892/ijmm.2017.3234. Epub 2017 Nov 3. Int J Mol Med. 2018. PMID: 29115400 Free PMC article.
-
ERK-mediated Cytoplasmic Retention of USP11 Contributes to Breast Cancer Cell Proliferation by Stabilizing Cytoplasmic p21.Int J Biol Sci. 2022 Mar 21;18(6):2568-2582. doi: 10.7150/ijbs.71327. eCollection 2022. Int J Biol Sci. 2022. PMID: 35414784 Free PMC article.
-
LncRNA-ZXF1 stabilizes P21 expression in endometrioid endometrial carcinoma by inhibiting ubiquitination-mediated degradation and regulating the miR-378a-3p/PCDHA3 axis.Mol Oncol. 2022 Feb;16(3):813-829. doi: 10.1002/1878-0261.12940. Epub 2021 Mar 22. Mol Oncol. 2022. PMID: 33751805 Free PMC article.
-
Alternative polyadenylation and dynamic 3' UTR length is associated with polysome recruitment throughout the cardiomyogenic differentiation of hESCs.Front Mol Biosci. 2024 Feb 6;11:1336336. doi: 10.3389/fmolb.2024.1336336. eCollection 2024. Front Mol Biosci. 2024. PMID: 38380430 Free PMC article.
References
-
- Sherr C. J., Roberts J. M. (1999) Genes Dev. 13, 1501–1512 - PubMed
-
- Gartel A. L., Tyner A. L. (1999) Exp. Cell Res. 246, 280–289 - PubMed
-
- Duli V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. (1994) Cell 76, 1013–1023 - PubMed
-
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. (1993) Cell 75, 817–825 - PubMed
-
- Bendjennat M., Boulaire J., Jascur T., Brickner H., Barbier V., Sarasin A., Fotedar A., Fotedar R. (2003) Cell 114, 599–610 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

