miR-183-96-182 cluster is overexpressed in prostate tissue and regulates zinc homeostasis in prostate cells
- PMID: 22045813
- PMCID: PMC3247959
- DOI: 10.1074/jbc.M111.262915
miR-183-96-182 cluster is overexpressed in prostate tissue and regulates zinc homeostasis in prostate cells
Abstract
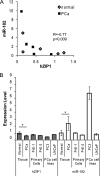
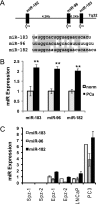
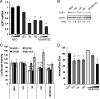
Decreased zinc levels are a hallmark of prostate cancer tumors as zinc uniquely concentrates in healthy prostate tissue. Increased dietary zinc correlates with decreased risk of advanced prostate cancer and decreased mortality from prostate cancer. The mechanisms of prostatic zinc homeostasis are not known. Lower zinc levels in the tumor are correlated directly with decreased expression of the zinc transporter hZIP1. We report identification of a microRNA cluster that regulates multiple zinc transporters, including hZIP1. Screening in laser capture microdissected prostate cancer tumors identified miR-182 as a potential regulator of hZIP1. Regulation of hZIP1 by miR-182 via two binding sites was confirmed in primary prostate cell cultures. miR-96 and miR-183 are expressed as a cluster with miR-182 and share similar sequences. Array profiling of tissue showed that miR-183, -96, and -182 are higher in prostate cancer tissue compared with normal prostate. Overexpression of the entire miR-183-96-182 cluster suppressed five additional zinc transporters. Overexpression of miR-183, -96, and -182 individually or as a cluster diminished labile zinc pools and reduced zinc uptake, demonstrating this miR cluster as a regulator of zinc homeostasis. We observed regulation of zinc homeostasis by this cluster in prostate cells and HEK-293 cells, suggesting a universal mechanism that is not prostate-specific. To our knowledge, this is the first report of a miR cluster targeting a family of metal transport proteins. Individually or as a cluster, miR-183, -96, and -182 are overexpressed in other cancers too, implicating this miR cluster in carcinogenesis.
Figures
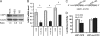
References
-
- Costello L. C., Franklin R. B., Feng P., Tan M., Bagasra O. (2005) Cancer Causes Control 16, 901–915 - PubMed
-
- Mawson C. A., Fischer M. I. (1952) Can J. Med. Sci. 30, 336–339 - PubMed
-
- Cortesi M., Fridman E., Volkov A., Shilstein SSh., Chechik R., Breskin A., Vartsky D., Kleinman N., Kogan G., Moriel E., Gladysh V., Huszar M., Ramon J., Raviv G. (2008) Prostate 68, 994–1006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

