Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1
- PMID: 22045868
- PMCID: PMC3290424
- DOI: 10.1189/jlb.0611308
Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1
Abstract
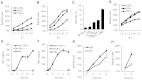
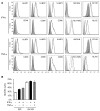
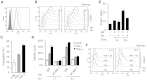
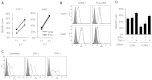
NK cells control tumor and virus-infected cells through releasing cytotoxic granules and proinflammatory cytokines. IFN-γ and TNF-α secretions and cytotoxicity are regarded as two distinct functions of NK cells with little synergy in between as results of early association of the two functions with distinct subsets of NK populations and of the studies showing target cells developing NK resistance upon IFN-γ treatment. Here, we show that IFN-γ and TNF-α synergistically enhance NK cell cytotoxicity through NF-κB-dependent up-regulation of ICAM-1 expression in target cells, thereby promoting their conjugate formation with NK cells. Neutralizing IFN-γ and TNF-α during cytolysis significantly impaired NK cell lysis of the target cells. Further, tumor cells exhibiting IFN-γ-inducible lysis are generally less-sensitive NK target cells but express inducible levels of ICAM-1. In contrast, sensitive NK targets tend to express higher but less-inducible ICAM-1. Their preferential induction in the lysis of insensitive NK target cells suggests that IFN-γ and TNF-α are functionally linked to and should be regarded as an integral part of NK cytolytic function.
Figures


References
-
- Biron C. A., Nguyen K. B., Pien G. C., Cousens L. P., Salazar-Mather T. P. (1999) Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 - PubMed
-
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. (1978) Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature 273, 759–761 - PubMed
-
- Trinchieri G., Santoli D., Koprowski H. (1978) Spontaneous cell-mediated cytotoxicity in humans: role of interferon and immunoglobulins. J. Immunol. 120, 1849–1855 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

