Molecular determinants of pentamidine-induced hERG trafficking inhibition
- PMID: 22046004
- PMCID: PMC3263949
- DOI: 10.1124/mol.111.075135
Molecular determinants of pentamidine-induced hERG trafficking inhibition
Abstract
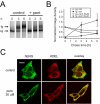
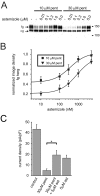
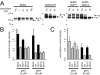
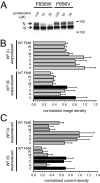
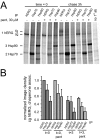
Pentamidine is an antiprotozoal compound that clinically causes acquired long QT syndrome (acLQTS), which is associated with prolonged QT intervals, tachycardias, and sudden cardiac arrest. Pentamidine delays terminal repolarization in human heart by acutely blocking cardiac inward rectifier currents. At the same time, pentamidine reduces surface expression of the cardiac potassium channel I(Kr)/human ether à-go-go-related gene (hERG). This is unusual in that acLQTS is caused most often by direct block of the cardiac potassium current I(Kr)/hERG. The present study was designed to provide a more complete picture of how hERG surface expression is disrupted by pentamidine at the cellular and molecular levels. Using biochemical and electrophysiological methods, we found that pentamidine exclusively inhibits hERG export from the endoplasmic reticulum to the cell surface in a heterologous expression system as well as in cardiomyocytes. hERG trafficking inhibition could be rescued in the presence of the pharmacological chaperone astemizole. We used rescue experiments in combination with an extensive mutational analysis to locate an interaction site for pentamidine at phenylalanine 656, a crucial residue in the canonical drug binding site of terminally folded hERG. Our data suggest that pentamidine binding to a folding intermediate of hERG arrests channel maturation in a conformational state that cannot be exported from the endoplasmic reticulum. We propose that pentamidine is the founding member of a novel pharmacological entity whose members act as small molecule antichaperones.
Figures

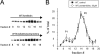

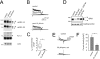
References
-
- Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. (2006) Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113:365–373 - PubMed
-
- Barbey JT, Pezzullo JC, Soignet SL. (2003) Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol 21:3609–3615 - PubMed
-
- Bian JS, Cui J, Melman Y, McDonald TV. (2004) S641 contributes HERG K+ channel inactivation. Cell Biochem Biophys 41:25–40 - PubMed
-
- Bibler MR, Chou TC, Toltzis RJ, Wade PA. (1988) Recurrent ventricular tachycardia due to pentamidine-induced cardiotoxicity. Chest 94:1303–1306 - PubMed
-
- Burchmore RJ, Ogbunude PO, Enanga B, Barrett MP. (2002) Chemotherapy of human African trypanosomiasis. Curr Pharm Des 8:256–267 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

