Speech production as state feedback control
- PMID: 22046152
- PMCID: PMC3200525
- DOI: 10.3389/fnhum.2011.00082
Speech production as state feedback control
Abstract
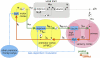
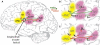
Spoken language exists because of a remarkable neural process. Inside a speaker's brain, an intended message gives rise to neural signals activating the muscles of the vocal tract. The process is remarkable because these muscles are activated in just the right way that the vocal tract produces sounds a listener understands as the intended message. What is the best approach to understanding the neural substrate of this crucial motor control process? One of the key recent modeling developments in neuroscience has been the use of state feedback control (SFC) theory to explain the role of the CNS in motor control. SFC postulates that the CNS controls motor output by (1) estimating the current dynamic state of the thing (e.g., arm) being controlled, and (2) generating controls based on this estimated state. SFC has successfully predicted a great range of non-speech motor phenomena, but as yet has not received attention in the speech motor control community. Here, we review some of the key characteristics of speech motor control and what they say about the role of the CNS in the process. We then discuss prior efforts to model the role of CNS in speech motor control, and argue that these models have inherent limitations - limitations that are overcome by an SFC model of speech motor control which we describe. We conclude by discussing a plausible neural substrate of our model.
Keywords: models of neural processes; models of speech production; sensory feedback; speech motor control; speech neurophysiology.
Figures
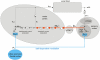

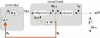
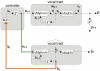
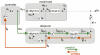
References
-
- Abbs J. H., Gracco V. L. (1983). Sensorimotor actions in the control of multi-movement speech gestures. Trends Neurosci. 6, 391.10.1016/0166-2236(83)90173-X - DOI
-
- Abbs J. H., Gracco V. L. (1984). Control of complex motor gestures: orofacial muscle responses to load perturbations of lip during speech. J. Neurophysiol. 51, 705–723 - PubMed
-
- Arbib M. A. (1981). “Perceptual structures and distributed motor control,” in Handbook of Physiology, Section 1: The Nervous System, Volume 2: Motor Control, Part 2, eds Brookhart J. M., Mountcastle V. B., Brooks V. B. (Bethesda, MD: American Phsyiological Society; ), 1449–1480

