Quantification of lateral heterogeneity in carbohydrate permeability of isolated plant leaf cuticles
- PMID: 22046169
- PMCID: PMC3202220
- DOI: 10.3389/fmicb.2011.00197
Quantification of lateral heterogeneity in carbohydrate permeability of isolated plant leaf cuticles
Abstract
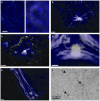
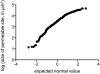
In phyllosphere microbiology, the distribution of resources available to bacterial colonizers of leaf surfaces is generally understood to be very heterogeneous. However, there is little quantitative understanding of the mechanisms that underlie this heterogeneity. Here, we tested the hypothesis that different parts of the cuticle vary in the degree to which they allow diffusion of the leaf sugar fructose to the surface. To this end, individual, isolated cuticles of poplar leaves were each analyzed for two properties: (1) the permeability for fructose, which involved measurement of diffused fructose by gas chromatography and flame ionization detection (GC-FID), and (2) the number and size of fructose-permeable sites on the cuticle, which was achieved using a green-fluorescent protein (GFP)-based bacterial bioreporter for fructose. Bulk flux measurements revealed an average permeance P of 3.39 × 10(-9) ms(-1), while the bioreporter showed that most of the leaching fructose was clustered to sites around the base of shed trichomes, which accounted for only 0.37% of the surface of the cuticles under study. Combined, the GC-FID and GFP measurements allowed us to calculate an apparent rate of fructose diffusion at these preferential leaching sites of 9.15 × 10(-7) ms(-1). To the best of our knowledge, this study represents the first successful attempt to quantify cuticle permeability at a resolution that is most relevant to bacterial colonizers of plant leaves. The estimates for P at different spatial scales will be useful for future models that aim to explain and predict temporal and spatial patterns of bacterial colonization of plant foliage based on lateral heterogeneity in sugar permeability of the leaf cuticle.
Keywords: Erwinia herbicola; Populus x canescens; aqueous pores; fructose; gas chromatography; phyllosphere; poplar.
Figures

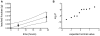
Similar articles
-
Modelling sugar diffusion across plant leaf cuticles: the effect of free water on substrate availability to phyllosphere bacteria.Environ Microbiol. 2011 Mar;13(3):792-7. doi: 10.1111/j.1462-2920.2010.02382.x. Epub 2010 Nov 22. Environ Microbiol. 2011. PMID: 21091864
-
Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere.Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3446-53. doi: 10.1073/pnas.061629598. Proc Natl Acad Sci U S A. 2001. PMID: 11248098 Free PMC article.
-
A new technique for measurement of water permeability of stomatous cuticular membranes isolated from Hedera helix leaves.J Exp Bot. 2004 Jun;55(401):1411-22. doi: 10.1093/jxb/erh150. Epub 2004 May 21. J Exp Bot. 2004. PMID: 15155780
-
Characterization of aqueous pores in plant cuticles and permeation of ionic solutes.J Exp Bot. 2006;57(11):2471-91. doi: 10.1093/jxb/erj217. Epub 2006 Jul 6. J Exp Bot. 2006. PMID: 16825315 Review.
-
Polar paths of diffusion across plant cuticles: new evidence for an old hypothesis.Ann Bot. 2005 Jun;95(7):1069-73. doi: 10.1093/aob/mci122. Epub 2005 Mar 29. Ann Bot. 2005. PMID: 15797897 Free PMC article. Review.
Cited by
-
Driving factors of epiphytic bacterial communities: A review.J Adv Res. 2019 Mar 14;19:57-65. doi: 10.1016/j.jare.2019.03.003. eCollection 2019 Sep. J Adv Res. 2019. PMID: 31341670 Free PMC article. Review.
-
Chemical and structural analysis of Eucalyptus globulus and E. camaldulensis leaf cuticles: a lipidized cell wall region.Front Plant Sci. 2014 Sep 16;5:481. doi: 10.3389/fpls.2014.00481. eCollection 2014. Front Plant Sci. 2014. PMID: 25278953 Free PMC article.
-
The Chemical Landscape of Leaf Surfaces and Its Interaction with the Atmosphere.Chem Rev. 2024 May 8;124(9):5764-5794. doi: 10.1021/acs.chemrev.3c00763. Epub 2024 Apr 23. Chem Rev. 2024. PMID: 38652704 Free PMC article. Review.
-
Plant species-dependent transmission of Escherichia coli O157:H7 from the spermosphere to cotyledons and first leaves.Environ Microbiol Rep. 2022 Dec;14(6):926-933. doi: 10.1111/1758-2229.13115. Epub 2022 Aug 15. Environ Microbiol Rep. 2022. PMID: 35968609 Free PMC article.
-
From Genes to Ecosystems in Microbiology: Modeling Approaches and the Importance of Individuality.Front Microbiol. 2017 Nov 27;8:2299. doi: 10.3389/fmicb.2017.02299. eCollection 2017. Front Microbiol. 2017. PMID: 29230200 Free PMC article. Review.
References
-
- Abramoff M. D., Magelhaes P. J., Ram S. J. (2004). Image processing with imageJ. Biophotonics Int. 11, 36–42
-
- Benedict S. R. (1909). A reagent for the detection of reducing sugars. J. Biol. Chem. 5, 485 - PubMed
-
- Bradshaw H. D., Ceulemans R., Davis J., Stettler R. (2000). Emerging model systems in plant biology: poplar (populus) as a model forest tree. J. Plant Growth Regul. 19, 306–31310.1007/s003440000030 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

