Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis
- PMID: 22046219
- PMCID: PMC3205809
Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis
Abstract
Background: Although there is general agreement that metformin should be used as first-line pharmacotherapy in patients with type 2 diabetes, uncertainty remains regarding the choice of second-line therapy once metformin is no longer effective. We conducted a systematic review and meta-analysis to assess the comparative safety and efficacy of all available classes of antihyperglycemic therapies in patients with type 2 diabetes inadequately controlled on metformin monotherapy.
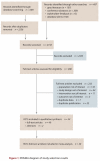
Methods: MEDLINE, EMBASE, BIOSIS Previews, PubMed and the Cochrane Central Register of Controlled Trials were searched for randomized controlled trials published in English from 1980 to October 2009. Additional citations were obtained from grey literature and conference proceedings and through stakeholder feedback. Two reviewers independently selected studies, extracted data and assessed risk of bias. Key outcomes of interest were hemoglobin A1c, body weight, hypoglycemia, quality of life, long-term diabetes-related complications, serious adverse drug events and mortality. Mixed-treatment comparison and pairwise meta-analyses were conducted to pool trial results, when appropriate.
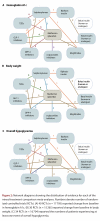
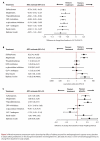
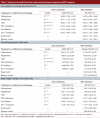
Results: We identified 49 active and non-active controlled randomized trials that compared 2 or more of the following classes of antihyperglycemic agents and weight-loss agents: sulfonylureas, meglitinides, thiazolidinediones (TZDs), dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) analogues, insulins, alpha-glucosidase inhibitors, sibutramine and orlistat. All classes of second-line antihyperglycemic therapies achieved clinically meaningful reductions in hemoglobin A1c (0.6% to 1.0%). No significant differences were found between classes. Insulins and insulin secretagogues were associated with significantly more events of overall hypoglycemia than the other agents, but severe hypoglycemia was rarely observed. An increase in body weight was observed with the majority of second-line therapies (1.8 to 3.0 kg), the exceptions being DPP-4 inhibitors, alpha-glucosidase inhibitors and GLP-1 analogues (0.6 to -1.8 kg). There were insufficient data available for diabetes complications, mortality or quality of life.
Interpretation: DPP-4 inhibitors and GLP-1 analogues achieved improvements in glycemic control similar to those of other second-line therapies, although they may have modest benefits in terms of weight gain and overall hypoglycemia. Further long-term trials of adequate power are required to determine whether newer drug classes differ from older agents in terms of clinically meaningful outcomes.
Conflict of interest statement
Competing interests: Marshall Dahl has received an honorarium of less than $5000 from Eli Lilly Canada Inc. for workshops. He has also received an arm’s length grant for a diabetes study in patients with coronary artery disease from GlaxoSmithKline Inc. Nicky Welton has received a contribution of £2250 from Pfizer PLC to provide a course on MTC methods.
Figures

Similar articles
-
Choice of therapy in patients with type 2 diabetes inadequately controlled with metformin and a sulphonylurea: a systematic review and mixed-treatment comparison meta-analysis.Open Med. 2012 Jun 4;6(2):e62-74. Print 2012. Open Med. 2012. PMID: 23696771 Free PMC article.
-
Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus.Cochrane Database Syst Rev. 2017 May 10;5(5):CD012204. doi: 10.1002/14651858.CD012204.pub2. Cochrane Database Syst Rev. 2017. PMID: 28489279 Free PMC article.
-
Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation.Health Technol Assess. 2010 Jul;14(36):1-248. doi: 10.3310/hta14360. Health Technol Assess. 2010. PMID: 20646668
-
Insulin and glucose-lowering agents for treating people with diabetes and chronic kidney disease.Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011798. doi: 10.1002/14651858.CD011798.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246878 Free PMC article.
-
Interventions for people with type 2 diabetes mellitus fasting during Ramadan.Cochrane Database Syst Rev. 2023 Jul 12;7(7):CD013178. doi: 10.1002/14651858.CD013178.pub2. Cochrane Database Syst Rev. 2023. PMID: 37435938 Free PMC article.
Cited by
-
Ten-Year Trends in the Morbidity of Diabetes Mellitus and Antidiabetic Drug Utilization in Croatia: A Study Based on Routinely Collected Data.Int J Family Med. 2016;2016:9837496. doi: 10.1155/2016/9837496. Epub 2016 Jul 4. Int J Family Med. 2016. PMID: 27462470 Free PMC article.
-
Comparison of acarbose and voglibose in diabetes patients who are inadequately controlled with basal insulin treatment: randomized, parallel, open-label, active-controlled study.J Korean Med Sci. 2014 Jan;29(1):90-7. doi: 10.3346/jkms.2014.29.1.90. Epub 2013 Dec 26. J Korean Med Sci. 2014. PMID: 24431911 Free PMC article. Clinical Trial.
-
Beyond glycated haemoglobin: Modelling contemporary management of type 2 diabetes with the updated Cardiff model.Diabetes Obes Metab. 2025 Apr;27(4):1752-1761. doi: 10.1111/dom.16141. Epub 2025 Jan 19. Diabetes Obes Metab. 2025. PMID: 39828939 Free PMC article.
-
Efficacy and safety of combination therapy with vildagliptin and metformin versus metformin up-titration in Chinese patients with type 2 diabetes mellitus: study design and rationale of the vision study.Cardiovasc Diabetol. 2013 Aug 19;12:118. doi: 10.1186/1475-2840-12-118. Cardiovasc Diabetol. 2013. PMID: 23958390 Free PMC article. Clinical Trial.
-
Hypoglycaemia when adding sulphonylurea to metformin: a systematic review and network meta-analysis.Br J Clin Pharmacol. 2016 Nov;82(5):1291-1302. doi: 10.1111/bcp.13059. Epub 2016 Aug 3. Br J Clin Pharmacol. 2016. PMID: 27426428 Free PMC article.
References
-
- Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. [2010-01-27];Can J Diabetes. 2008 32(Suppl 1):1–201. http://www.diabetes.ca/files/cpg2008/cpg-2008.pdf
-
- National Collaborating Centre for Chronic Conditions. Type 2 diabetes: National clinical guideline for management in primary and secondary care (update) London (UK): Royal College of Physicians; 2008. http://www.nice.org.uk/nicemedia/pdf/CG66diabetesfullguideline.pdf - PubMed
-
- Managing type 2 diabetes in south Australia. Adelaide: Government of South Australia, Department of Health; 2008. http://www.publications.health.sa.gov.au/cgi/viewcontent.cgi?article=100...
-
- Management of type 2 diabetes. Wellington: New Zealand Guidelines Group (NZGG); 2003. [accessed 2009 Jan 19]. http://www.nzgg.org.nz/guidelines/dsp_guideline_popup.cfm?guidelineCatID...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
