Increased feeding and food hoarding following food deprivation are associated with activation of dopamine and orexin neurons in male Brandt's voles
- PMID: 22046281
- PMCID: PMC3203142
- DOI: 10.1371/journal.pone.0026408
Increased feeding and food hoarding following food deprivation are associated with activation of dopamine and orexin neurons in male Brandt's voles
Abstract
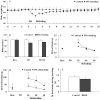
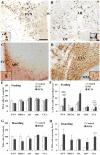
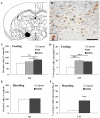
Small mammals usually face energetic challenges, such as food shortage, in the field. They have thus evolved species-specific adaptive strategies for survival and reproductive success. In the present study, we examined male Brandt's voles (Lasiopodomys brandtii) for their physiological, behavioral, and neuronal responses to food deprivation (FD) and subsequent re-feeding. Although 48 hr FD induced a decrease in body weight and the resting metabolic rate (RMR), such decreases did not reach statistical significance when compared to the control males that did not experience FD. During the first 2 hr of re-feeding following 48 hr FD, voles showed higher levels of feeding than controls. However, when permitted to hoard food, FD voles showed an increase in food hoarding, rather than feeding, compared to the controls. Further, both feeding and food hoarding induced an increase in neuronal activation, measured by Fos-ir, in a large number of brain areas examined. Interestingly, feeding and food hoarding also induced an increase in the percentage of tyrosine hydroxylase immunoreactive (TH-ir) cells that co-expressed Fos-ir in the ventral tegmental area (VTA), whereas both FD and feeding induced an increase in the percentage of orexin-ir cells that co-expressed Fos-ir in the lateral hypothalamus (LH). Food hoarding also increased orexin-ir/Fos-ir labeling in the LH. Together, our data indicate that food-deprived male Brandt's voles display enhanced feeding or food hoarding dependent upon an environmental setting. In addition, changes in central dopamine and orexin activities in selected brain areas are associated with feeding and hoarding behaviors following FD and subsequent re-feeding.
Conflict of interest statement
Figures
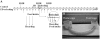

Similar articles
-
The Individual Division of Food Hoarding in Autumn Brandt's Voles (Lasiopodomys brandtii).Animals (Basel). 2024 Sep 20;14(18):2719. doi: 10.3390/ani14182719. Animals (Basel). 2024. PMID: 39335308 Free PMC article.
-
Food hoarding and associated neuronal activation in brain reward circuitry in Mongolian gerbils.Physiol Behav. 2011 Sep 1;104(3):429-36. doi: 10.1016/j.physbeh.2011.04.062. Epub 2011 May 5. Physiol Behav. 2011. PMID: 21570992
-
Re-feeding evokes reproductive overcompensation of food-restricted Brandt's voles.Physiol Behav. 2012 Feb 1;105(3):653-60. doi: 10.1016/j.physbeh.2011.09.026. Epub 2011 Oct 12. Physiol Behav. 2012. PMID: 22019786
-
Orexins and orexin receptors: implication in feeding behavior.Regul Pept. 1999 Nov 30;85(1):25-30. doi: 10.1016/s0167-0115(99)00076-2. Regul Pept. 1999. PMID: 10588447 Review.
-
Roles of orexins and orexin receptors in central regulation of feeding behavior and energy homeostasis.CNS Neurol Disord Drug Targets. 2006 Jun;5(3):313-25. doi: 10.2174/187152706777452218. CNS Neurol Disord Drug Targets. 2006. PMID: 16787231 Review.
Cited by
-
An effort toward molecular neuroeconomics of food deprivation induced food hoarding in mice: focus on xanthine oxidoreductase gene expression and xanthine oxidase activity.Metab Brain Dis. 2018 Feb;33(1):325-331. doi: 10.1007/s11011-017-0166-2. Epub 2017 Dec 19. Metab Brain Dis. 2018. PMID: 29260359
-
Neuroendocrine regulation of appetitive ingestive behavior.Front Neurosci. 2013 Nov 15;7:213. doi: 10.3389/fnins.2013.00213. Front Neurosci. 2013. PMID: 24298235 Free PMC article. Review.
-
An anatomically distinct subpopulation of orexin neurons project from the lateral hypothalamus to the olfactory bulb.J Comp Neurol. 2023 Oct;531(15):1510-1524. doi: 10.1002/cne.25518. Epub 2023 Jul 11. J Comp Neurol. 2023. PMID: 37434469 Free PMC article.
-
Cocaine- and amphetamine-regulated transcript peptide- and dopamine-containing systems interact in the ventral tegmental area of the zebra finch, Taeniopygia guttata, during dynamic changes in energy status.Brain Struct Funct. 2021 Nov;226(8):2537-2559. doi: 10.1007/s00429-021-02348-y. Epub 2021 Aug 14. Brain Struct Funct. 2021. PMID: 34392422
References
-
- Nagashima Y, Ohno T, Ogawa K, Kuroshima A. Effects of fasting and refeeding on some metabolic characteristics of rat brown adipose tissue. Jpn J Physiol. 1995;45:645–658. - PubMed
-
- Hambly C, Speakman JR. Contribution of different mechanisms to compensation for energy restriction in the mouse. Obes Res. 2005;13:1548–1557. - PubMed
-
- Gutman R, Choshniak I, Kronfeld-Schor N. Defending body mass during food restriction in Acomys russatus: a desert rodent that does not store food. Am J Physiol Regul Integr Comp Physiol. 2006;290:R881–891. - PubMed
-
- Gutman R, Yosha D, Choshniak I, Kronfeld-Schor N. Two strategies for coping with food shortage in desert golden spiny mice. Physiol Behav. 2007;90:95–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

