Overexpression of active Aurora-C kinase results in cell transformation and tumour formation
- PMID: 22046298
- PMCID: PMC3203144
- DOI: 10.1371/journal.pone.0026512
Overexpression of active Aurora-C kinase results in cell transformation and tumour formation
Abstract
Aurora kinases belong to a conserved family of serine/threonine kinases key regulators of cell cycle progression. Aurora-A and Aurora-B are expressed in somatic cells and involved mainly in mitosis while Aurora-C is expressed during spermatogenesis and oogenesis and is involved in meiosis. Aurora-C is hardly detectable in normal somatic cells. However all three kinases are overexpressed in many cancer lines. Aurora-A possesses an oncogenic activity while Aurora-B does not. Here we investigated whether Aurora-C possesses such an oncogenic activity. We report that overexpression of Aurora-C induces abnormal cell division resulting in centrosome amplification and multinucleation in both transiently transfected cells and in stable cell lines. Only stable NIH3T3 cell clones overexpressing active Aurora-C formed foci of colonies when grown on soft agar, indicating that a gain of Aurora-C activity is sufficient to transform cells. Furthermore, we reported that NIH-3T3 stable cell lines overexpressing Aurora-C induced tumour formation when injected into nude mice, demonstrating the oncogenic activity of enzymatically active Aurora kinase C. Interestingly enough tumor aggressiveness was positively correlated with the quantity of active kinase, making Aurora-C a potential anti-cancer therapeutic target.
Conflict of interest statement
Figures
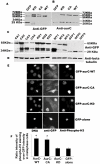


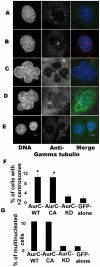

References
-
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. - PubMed
-
- Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, et al. Multinuclearity and increased ploidy caused by overexpression of the Aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998;58:4811–4816. - PubMed
-
- Kimura M, Matsuda Y, Yoshioka T, Okano Y. Cell cycle-dependent expression and centrosome localization of a third human Aurora/Ipl1-related protein kinase, AIK3. J Biol Chem. 1999;274:7334–7340. - PubMed
-
- Sasai K, Katayama H, Stenoien DL, Fuji S, Honda R, et al. Aurora-C kinase is a novel chromosomal passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton. 2004;59:249–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

