Characterization of protection afforded by a bivalent virus-like particle vaccine against bluetongue virus serotypes 1 and 4 in sheep
- PMID: 22046324
- PMCID: PMC3202233
- DOI: 10.1371/journal.pone.0026666
Characterization of protection afforded by a bivalent virus-like particle vaccine against bluetongue virus serotypes 1 and 4 in sheep
Abstract
Background: Bluetongue virus (BTV) is an economically important, arthropod borne, emerging pathogen in Europe, causing disease mainly in sheep and cattle. Routine vaccination for bluetongue would require the ability to distinguish between vaccinated and infected individuals (DIVA). Current vaccines are effective but are not DIVA. Virus-like particles (VLPs) are highly immunogenic structural mimics of virus particles, that only contain a subset of the proteins present in a natural infection. VLPs therefore offer the potential for the development of DIVA compatible bluetongue vaccines.
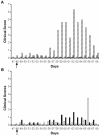
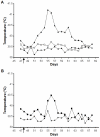
Methodology/principal findings: Merino sheep were vaccinated with either monovalent BTV-1 VLPs or a bivalent mixture of BTV-1 VLPs and BTV-4 VLPs, and challenged with virulent BTV-1 or BTV-4. Animals were monitored for clinical signs, antibody responses, and viral RNA. 19/20 animals vaccinated with BTV-1 VLPs either alone or in combination with BTV-4 VLPs developed neutralizing antibodies to BTV-1, and group specific antibodies to BTV VP7. The one animal that showed no detectable neutralizing antibodies, or group specific antibodies, had detectable viral RNA following challenge but did not display any clinical signs on challenge with virulent BTV-1. In contrast, all control animals' demonstrated classical clinical signs for bluetongue on challenge with the same virus. Six animals were vaccinated with bivalent vaccine and challenged with virulent BTV-4, two of these animals had detectable viral levels of viral RNA, and one of these showed clinical signs consistent with BTV infection and died.
Conclusions: There is good evidence that BTV-1 VLPs delivered as monovalent or bivalent immunogen protect from bluetongue disease on challenge with virulent BTV-1. However, it is possible that there is some interference in protective response for BTV-4 in the bivalent BTV-1 and BTV-4 VLP vaccine. This raises the question of whether all combinations of bivalent BTV vaccines are possible, or if immunodominance of particular serotypes could interfere with vaccine efficacy.
Conflict of interest statement
Figures



Similar articles
-
Bluetongue virus serotype 8 virus-like particles protect sheep against virulent virus infection as a single or multi-serotype cocktail immunogen.Vaccine. 2013 Jan 7;31(3):553-8. doi: 10.1016/j.vaccine.2012.11.016. Epub 2012 Nov 14. Vaccine. 2013. PMID: 23159460
-
Plant-produced Bluetongue chimaeric VLP vaccine candidates elicit serotype-specific immunity in sheep.Vaccine. 2019 Sep 24;37(41):6068-6075. doi: 10.1016/j.vaccine.2019.08.042. Epub 2019 Aug 27. Vaccine. 2019. PMID: 31471154
-
Protective efficacy of Bluetongue virus-like and subvirus-like particles in sheep: presence of the serotype-specific VP2, independent of its geographic lineage, is essential for protection.Vaccine. 2012 Mar 9;30(12):2131-9. doi: 10.1016/j.vaccine.2012.01.042. Epub 2012 Jan 26. Vaccine. 2012. PMID: 22285887
-
Nature and duration of protective immunity to bluetongue virus infection.Dev Biol (Basel). 2003;114:169-83. Dev Biol (Basel). 2003. PMID: 14677687 Review.
-
Vaccines for bluetongue.Aust Vet J. 1996 Jun;73(6):207-10. doi: 10.1111/j.1751-0813.1996.tb10036.x. Aust Vet J. 1996. PMID: 8893989 Review.
Cited by
-
Assessment of Pain and Inflammation in Domestic Animals Using Infrared Thermography: A Narrative Review.Animals (Basel). 2023 Jun 22;13(13):2065. doi: 10.3390/ani13132065. Animals (Basel). 2023. PMID: 37443863 Free PMC article. Review.
-
A review of experimental infections with bluetongue virus in the mammalian host.Virus Res. 2014 Mar;182:21-34. doi: 10.1016/j.virusres.2013.12.044. Epub 2014 Jan 24. Virus Res. 2014. PMID: 24462840 Free PMC article. Review.
-
Development of plant-produced protein body vaccine candidates for bluetongue virus.BMC Biotechnol. 2017 May 30;17(1):47. doi: 10.1186/s12896-017-0370-5. BMC Biotechnol. 2017. PMID: 28558675 Free PMC article.
-
Biomimetic Apatite Nanoparticles and Microcrystalline Tyrosine as Biocompatible Vaccine Adjuvants: Performance in a Bluetongue Virus Sheep Model.ACS Appl Mater Interfaces. 2025 Aug 13;17(32):45538-45554. doi: 10.1021/acsami.5c10402. Epub 2025 Jul 8. ACS Appl Mater Interfaces. 2025. PMID: 40626498 Free PMC article.
-
Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): bluetongue.EFSA J. 2017 Aug 4;15(8):e04957. doi: 10.2903/j.efsa.2017.4957. eCollection 2017 Aug. EFSA J. 2017. PMID: 32625623 Free PMC article.
References
-
- Roy P, Noad R. Bluetongue virus assembly and morphogenesis. Curr Top Microbiol Immunol. 2006;309:87–116. - PubMed
-
- Maclachlan NJ, Drew CP, Darpel KE, Worwa G. The pathology and pathogenesis of bluetongue. J Comp Pathol. 2009;141:1–16. - PubMed
-
- Schwartz-Cornil I, Mertens PP, Contreras V, Hemati B, Pascale F, et al. Bluetongue virus: virology, pathogenesis and immunity. Vet Res. 2008;39:46. - PubMed
-
- MacLachlan NJ, Crafford JE, Vernau W, Gardner IA, Goddard A, et al. Experimental reproduction of severe bluetongue in sheep. Vet Pathol. 2008;45:310–315. - PubMed
-
- Chaignat V, Worwa G, Scherrer N, Hilbe M, Ehrensperger F, et al. Toggenburg Orbivirus, a new bluetongue virus: initial detection, first observations in field and experimental infection of goats and sheep. Vet Microbiol. 2009;138:11–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

