N-cofilin can compensate for the loss of ADF in excitatory synapses
- PMID: 22046357
- PMCID: PMC3203908
- DOI: 10.1371/journal.pone.0026789
N-cofilin can compensate for the loss of ADF in excitatory synapses
Abstract
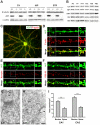
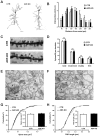
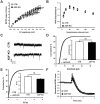
Actin plays important roles in a number of synaptic processes, including synaptic vesicle organization and exocytosis, mobility of postsynaptic receptors, and synaptic plasticity. However, little is known about the mechanisms that control actin at synapses. Actin dynamics crucially depend on LIM kinase 1 (LIMK1) that controls the activity of the actin depolymerizing proteins of the ADF/cofilin family. While analyses of mouse mutants revealed the importance of LIMK1 for both pre- and postsynaptic mechanisms, the ADF/cofilin family member n-cofilin appears to be relevant merely for postsynaptic plasticity, and not for presynaptic physiology. By means of immunogold electron microscopy and immunocytochemistry, we here demonstrate the presence of ADF (actin depolymerizing factor), a close homolog of n-cofilin, in excitatory synapses, where it is particularly enriched in presynaptic terminals. Surprisingly, genetic ablation of ADF in mice had no adverse effects on synapse structure or density as assessed by electron microscopy and by the morphological analysis of Golgi-stained hippocampal pyramidal cells. Moreover, a series of electrophysiological recordings in acute hippocampal slices revealed that presynaptic recruitment and exocytosis of synaptic vesicles as well as postsynaptic plasticity were unchanged in ADF mutant mice. The lack of synaptic defects may be explained by the elevated n-cofilin levels observed in synaptic structures of ADF mutants. Indeed, synaptic actin regulation was impaired in compound mutants lacking both ADF and n-cofilin, but not in ADF single mutants. From our results we conclude that n-cofilin can compensate for the loss of ADF in excitatory synapses. Further, our data suggest that ADF and n-cofilin cooperate in controlling synaptic actin content.
Conflict of interest statement
Figures
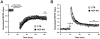

References
-
- Landis DM, Hall AK, Weinstein LA, Reese TS. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1:201–209. - PubMed
-
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

