Atomic-level characterization of the activation mechanism of SERCA by calcium
- PMID: 22046418
- PMCID: PMC3203174
- DOI: 10.1371/journal.pone.0026936
Atomic-level characterization of the activation mechanism of SERCA by calcium
Abstract
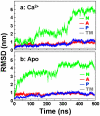
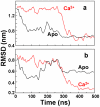
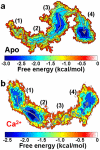
We have performed molecular dynamics (MD) simulations to elucidate, in atomic detail, the mechanism by which the sarcoplasmic reticulum Ca(2+)-ATPase (SERCA) is activated by Ca(2+). Crystal structures suggest that activation of SERCA occurs when the cytoplasmic head-piece, in an open (E1) conformation stabilized by Ca(2+), undergoes a large-scale open-to-closed (E1 to E2) transition that is induced by ATP binding. However, spectroscopic measurements in solution suggest that these structural states (E1 and E2) are not tightly coupled to biochemical states (defined by bound ligands); the closed E2 state predominates even in the absence of ATP, in both the presence and absence of Ca(2+). How is this loose coupling consistent with the high efficiency of energy transduction in the Ca(2+)-ATPase? To provide insight into this question, we performed long (500 ns) all-atom MD simulations starting from the open crystal structure, including a lipid bilayer and water. In both the presence and absence of Ca(2+), we observed a large-scale open-to-closed conformational transition within 400 ns, supporting the weak coupling between structural and biochemical states. However, upon closer inspection, it is clear that Ca(2+) is necessary and sufficient for SERCA to reach the precise geometrical arrangement necessary for activation of ATP hydrolysis. Contrary to suggestions from crystal structures, but in agreement with solution spectroscopy, the presence of ATP is not required for this activating transition. Principal component analysis showed that Ca(2+) reshapes the free energy landscape of SERCA to create a path between the open conformation and the activated closed conformation. Thus the malleability of the free energy landscape is essential for SERCA efficiency, ensuring that ATP hydrolysis is tightly coupled to Ca(2+) transport. These results demonstrate the importance of real-time dynamics in the formation of catalytically competent conformations of SERCA, with broad implications for understanding enzymatic catalysis in atomic detail.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

