Altering host resistance to infections through microbial transplantation
- PMID: 22046427
- PMCID: PMC3203939
- DOI: 10.1371/journal.pone.0026988
Altering host resistance to infections through microbial transplantation
Abstract
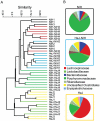
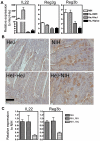
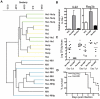
Host resistance to bacterial infections is thought to be dictated by host genetic factors. Infections by the natural murine enteric pathogen Citrobacter rodentium (used as a model of human enteropathogenic and enterohaemorrhagic E. coli infections) vary between mice strains, from mild self-resolving colonization in NIH Swiss mice to lethality in C3H/HeJ mice. However, no clear genetic component had been shown to be responsible for the differences observed with C. rodentium infections. Because the intestinal microbiota is important in regulating resistance to infection, and microbial composition is dependent on host genotype, it was tested whether variations in microbial composition between mouse strains contributed to differences in "host" susceptibility by transferring the microbiota of resistant mice to lethally susceptible mice prior to infection. Successful transfer of the microbiota from resistant to susceptible mice resulted in delayed pathogen colonization and mortality. Delayed mortality was associated with increased IL-22 mediated innate defense including antimicrobial peptides Reg3γ and Reg3β, and immunono-neutralization of IL-22 abrogated the beneficial effect of microbiota transfer. Conversely, depletion of the native microbiota in resistant mice by antibiotics and transfer of the susceptible mouse microbiota resulted in reduced innate defenses and greater pathology upon infection. This work demonstrates the importance of the microbiota and how it regulates mucosal immunity, providing an important factor in susceptibility to enteric infection. Transfer of resistance through microbial transplantation (bacteriotherapy) provides additional mechanisms to alter "host" resistance, and a novel means to alter enteric infection and to study host-pathogen interactions.
Conflict of interest statement
Figures
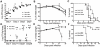


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

