Differential Secondary Reconstitution of In Vivo-Selected Human SCID-Repopulating Cells in NOD/SCID versus NOD/SCID/γ chain Mice
- PMID: 22046557
- PMCID: PMC3200073
- DOI: 10.1155/2011/252953
Differential Secondary Reconstitution of In Vivo-Selected Human SCID-Repopulating Cells in NOD/SCID versus NOD/SCID/γ chain Mice
Abstract
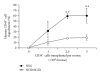
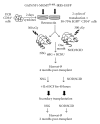
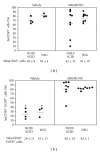
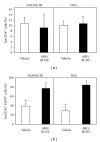
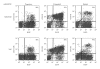
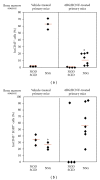
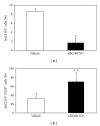
Humanized bone-marrow xenograft models that can monitor the long-term impact of gene-therapy strategies will help facilitate evaluation of clinical utility. The ability of the murine bone-marrow microenvironment in NOD/SCID versus NOD/SCID/γ chain(null) mice to support long-term engraftment of MGMT(P140K)-transduced human-hematopoietic cells following alkylator-mediated in vivo selection was investigated. Mice were transplanted with MGMT(P140K)-transduced CD34(+) cells and transduced cells selected in vivo. At 4 months after transplantation, levels of human-cell engraftment, and MGMT(P140K)-transduced cells in the bone marrow of NOD/SCID versus NSG mice varied slightly in vehicle- and drug-treated mice. In secondary transplants, although equal numbers of MGMT(P140K)-transduced human cells were transplanted, engraftment was significantly higher in NOD/SCID/γ chain(null) mice compared to NOD/SCID mice at 2 months after transplantation. These data indicate that reconstitution of NOD/SCID/γ chain(null) mice with human-hematopoietic cells represents a more promising model in which to test for genotoxicity and efficacy of strategies that focus on manipulation of long-term repopulating cells of human origin.
Figures

Similar articles
-
In vivo selection of MGMT(P140K) lentivirus-transduced human NOD/SCID repopulating cells without pretransplant irradiation conditioning.J Clin Invest. 2003 Nov;112(10):1561-70. doi: 10.1172/JCI17922. J Clin Invest. 2003. PMID: 14617757 Free PMC article.
-
In vivo selection of hematopoietic stem cells transduced at a low multiplicity-of-infection with a foamy viral MGMT(P140K) vector.Exp Hematol. 2008 Mar;36(3):283-92. doi: 10.1016/j.exphem.2007.11.009. Exp Hematol. 2008. PMID: 18279716 Free PMC article.
-
Hematopoietic expression of O(6)-methylguanine DNA methyltransferase-P140K allows intensive treatment of human glioma xenografts with combination O(6)-benzylguanine and 1,3-bis-(2-chloroethyl)-1-nitrosourea.Mol Cancer Ther. 2003 Dec;2(12):1321-9. Mol Cancer Ther. 2003. PMID: 14707273
-
Highly efficient transduction of the green fluorescent protein gene in human umbilical cord blood stem cells capable of cobblestone formation in long-term cultures and multilineage engraftment of immunodeficient mice.Blood. 1998 Dec 1;92(11):4013-22. Blood. 1998. PMID: 9834203
-
Efficient transduction of human hematopoietic repopulating cells generating stable engraftment of transgene-expressing cells in NOD/SCID mice.Blood. 2000 May 15;95(10):3085-93. Blood. 2000. PMID: 10807773
Cited by
-
In vivo enrichment of genetically manipulated platelets corrects the murine hemophilic phenotype and induces immune tolerance even using a low multiplicity of infection.J Thromb Haemost. 2014 Aug;12(8):1283-93. doi: 10.1111/jth.12633. Epub 2014 Jul 17. J Thromb Haemost. 2014. PMID: 24931217 Free PMC article.
-
Efficiency and safety of O⁶-methylguanine DNA methyltransferase (MGMT(P140K))-mediated in vivo selection in a humanized mouse model.Hum Gene Ther. 2014 Feb;25(2):144-55. doi: 10.1089/hum.2013.171. Epub 2014 Jan 7. Hum Gene Ther. 2014. PMID: 24218991 Free PMC article.
-
HIV-1 and drug abuse comorbidity: Lessons learned from the animal models of NeuroHIV.Neurosci Lett. 2021 May 29;754:135863. doi: 10.1016/j.neulet.2021.135863. Epub 2021 Mar 29. Neurosci Lett. 2021. PMID: 33794296 Free PMC article. Review.
-
How old is too old? In vivo engraftment of human peripheral blood stem cells cryopreserved for up to 18 years - implications for clinical transplantation and stability programs.World J Stem Cells. 2020 May 26;12(5):359-367. doi: 10.4252/wjsc.v12.i5.359. World J Stem Cells. 2020. PMID: 32547684 Free PMC article.
-
Measles virus envelope pseudotyped lentiviral vectors transduce quiescent human HSCs at an efficiency without precedent.Blood Adv. 2017 Oct 24;1(23):2088-2104. doi: 10.1182/bloodadvances.2017007773. eCollection 2017 Oct 24. Blood Adv. 2017. PMID: 29296856 Free PMC article.
References
-
- Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296(5577):2410–2413. - PubMed
-
- Aiuti A, Vai S, Mortellaro A, et al. Immune reconstitution in ADA-SCID after PBL gene therapy and discontinuation of enzyme replacement . Nature Medicine. 2002;8(5):423–425. - PubMed
-
- Cavazzana-Calvo M, Hacein-Bey S, de Saint-Basile G, Le Deist F, Fischer A. Gene therapy of severe combined immunodeficiencies. Transfusion Clinique et Biologique. 2000;7:259–260. - PubMed
-
- Cavazzana-Calvo M, Hacein-Bey S, De Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–672. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
