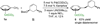Palladium-catalyzed allylic substitution with (η6-arene-CH2Z)Cr(CO)(3)-based nucleophiles
- PMID: 22047504
- PMCID: PMC3241867
- DOI: 10.1021/ja208935u
Palladium-catalyzed allylic substitution with (η6-arene-CH2Z)Cr(CO)(3)-based nucleophiles
Abstract
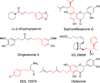
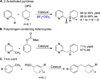
Although the palladium-catalyzed Tsuji-Trost allylic substitution reaction has been intensively studied, there is a lack of general methods to employ simple benzylic nucleophiles. Such a method would facilitate access to "α-2-propenyl benzyl" motifs, which are common structural motifs in bioactive compounds and natural products. We report herein the palladium-catalyzed allylation reaction of toluene-derived pronucleophiles activated by tricarbonylchromium. A variety of cyclic and acyclic allylic electrophiles can be employed with in situ generated (η(6)-C(6)H(5)CHLiR)Cr(CO)(3) nucleophiles. Catalyst identification was performed by high throughput experimentation (HTE) and led to the Xantphos/palladium hit, which proved to be a general catalyst for this class of reactions. In addition to η(6)-toluene complexes, benzyl amine and ether derivatives (η(6)-C(6)H(5)CH(2)Z)Cr(CO)(3) (Z = NR(2), OR) are also viable pronucleophiles, allowing C-C bond-formation α to heteroatoms with excellent yields. Finally, a tandem allylic substitution/demetalation procedure is described that affords the corresponding metal-free allylic substitution products. This method will be a valuable complement to the existing arsenal of nucleophiles with applications in allylic substitution reactions.
© 2011 American Chemical Society
Figures
References
-
- Cardenas DJ. Angew. Chem., Int. Ed. 2003;42:384. - PubMed
- Netherton MR, Fu GC. Adv. Synth. Catal. 2004;346:1525.
- Frisch AC, Beller M. Angew. Chem., Int. Ed. 2005;44:674. - PubMed
- Rudolph A, Lautens M. Angew. Chem., Int. Ed. 2009;48:2656. - PubMed
- Jana R, Pathak TP, Sigman MS. Chem. Rev. 2011;111:1417. - PMC - PubMed
-
- Trost BM, Van Vranken DL. Chem. Rev. 1996;96:395. - PubMed
- Trost BM, Crawley ML. Chem. Rev. 2003;103:2921. - PubMed
- Trost BM. J. Org. Chem. 2004;69:5813. - PubMed
- Trost BM, Machacek MR, Aponick A. Acc. Chem. Res. 2006;39:747. - PubMed
- Trost BM, Fandrick DR. Aldrichim. Acta. 2007;40:59.
- Lu Z, Ma S. Angew. Chem.. Int. Ed. 2008;47:258. - PubMed
-
- Castanet Y, Petit F. Tetrahedron Lett. 1979;34:3221.
- Keinan E, Roth Z. J. Org. Chem. 1983;48:1769. and references therein.
- Shukla KH, DeShong P. J. Org. Chem. 2008;73:6283. and references therein. - PubMed
-
- Dewick PM. Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal Chemistry and Biological Chemistry. Chichester, West Sussex, UK: John Wiley & Sons, Ltd; 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources