Inducible apoptosis as a safety switch for adoptive cell therapy
- PMID: 22047558
- PMCID: PMC3236370
- DOI: 10.1056/NEJMoa1106152
Inducible apoptosis as a safety switch for adoptive cell therapy
Abstract
Background: Cellular therapies could play a role in cancer treatment and regenerative medicine if it were possible to quickly eliminate the infused cells in case of adverse events. We devised an inducible T-cell safety switch that is based on the fusion of human caspase 9 to a modified human FK-binding protein, allowing conditional dimerization. When exposed to a synthetic dimerizing drug, the inducible caspase 9 (iCasp9) becomes activated and leads to the rapid death of cells expressing this construct.
Methods: We tested the activity of our safety switch by introducing the gene into donor T cells given to enhance immune reconstitution in recipients of haploidentical stem-cell transplants. Patients received AP1903, an otherwise bioinert small-molecule dimerizing drug, if graft-versus-host disease (GVHD) developed. We measured the effects of AP1903 on GVHD and on the function and persistence of the cells containing the iCasp9 safety switch.
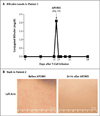
Results: Five patients between the ages of 3 and 17 years who had undergone stem-cell transplantation for relapsed acute leukemia were treated with the genetically modified T cells. The cells were detected in peripheral blood from all five patients and increased in number over time, despite their constitutive transgene expression. A single dose of dimerizing drug, given to four patients in whom GVHD developed, eliminated more than 90% of the modified T cells within 30 minutes after administration and ended the GVHD without recurrence.
Conclusions: The iCasp9 cell-suicide system may increase the safety of cellular therapies and expand their clinical applications. (Funded by the National Heart, Lung, and Blood Institute and the National Cancer Institute; ClinicalTrials.gov number, NCT00710892.).
Figures



Comment in
-
Eliminating cells gone astray.N Engl J Med. 2011 Nov 3;365(18):1735-7. doi: 10.1056/NEJMe1109971. N Engl J Med. 2011. PMID: 22047566 No abstract available.
References
-
- Daley GQ, Scadden DT. Prospects stem cell-based therapy. Cell. 2008;132:544–548. - PubMed
-
- Williams DA, Keating A. Enhancing research in regenerative medicine. Blood. 2010;116:866–867. - PubMed
-
- Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. - PubMed
-
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
