Microscopy in 3D: a biologist's toolbox
- PMID: 22047760
- PMCID: PMC3478882
- DOI: 10.1016/j.tcb.2011.09.008
Microscopy in 3D: a biologist's toolbox
Abstract
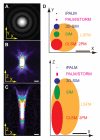
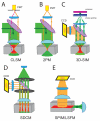
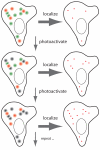
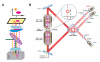
The power of fluorescence microscopy to study cellular structures and macromolecular complexes spans a wide range of size scales, from studies of cell behavior and function in physiological 3D environments to understanding the molecular architecture of organelles. At each length scale, the challenge in 3D imaging is to extract the most spatial and temporal resolution possible while limiting photodamage/bleaching to living cells. Several advances in 3D fluorescence microscopy now offer higher resolution, improved speed, and reduced photobleaching relative to traditional point-scanning microscopy methods. We discuss a few specific microscopy modalities that we believe will be particularly advantageous in imaging cells and subcellular structures in physiologically relevant 3D environments.
Published by Elsevier Ltd.
Figures
References
-
- Cheong W, et al. A review of the optical properties of biological tissues. IEEE J. Quantum Electron. 1990;26:2166–2185.
-
- Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010;7:603–614. - PubMed
-
- Conchello JA, Lichtman JW. Optical sectioning microscopy. Nat Methods. 2005;2:920–931. - PubMed
-
- Egner A, et al. Refractive index mismatch induced intensity and phase variations in fluorescence confocal, multiphoton and 4Pi-microscopy. Optics Communications. 1998;153:211–217.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

