A phase II study of gemcitabine in combination with tanespimycin in advanced epithelial ovarian and primary peritoneal carcinoma
- PMID: 22047770
- PMCID: PMC3265019
- DOI: 10.1016/j.ygyno.2011.10.002
A phase II study of gemcitabine in combination with tanespimycin in advanced epithelial ovarian and primary peritoneal carcinoma
Abstract
Objective: To evaluate the efficacy and biological effects of the gemcitabine/tanespimycin combination in patients with advanced ovarian and peritoneal cancer. To assess the effect of tanespimycin on tumor cells, levels of the chaperone proteins HSP90 and HSP70 were examined in peripheral blood mononuclear cells (PBMC) and paired tumor biopsy lysates.
Methods: Two-cohort phase II clinical trial. Patients were grouped according to prior gemcitabine therapy. All participants received tanespimycin 154 mg/m(2) on days 1 and 9 of cycle 1 and days 2 and 9 of subsequent cycles. Patients also received gemcitabine 750 mg/m(2) on day 8 of the first treatment cycle and days 1 and 8 of subsequent cycles.
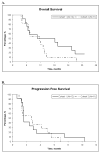
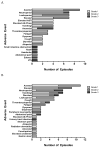
Results: The tanespimycin/gemcitabine combination induced a partial response in 1 gemcitabine naïve patient and no partial responses in gemcitabine resistant patients. Stable disease was seen in 6 patients (2 gemcitabine naïve and 4 gemcitabine resistant). The most common toxicities were hematologic (anemia and neutropenia) as well as nausea and vomiting. Immunoblotting demonstrated limited upregulation of HSP70 but little or no change in levels of most client proteins in PBMC and paired tumor samples.
Conclusions: Although well tolerated, the tanespimycin/gemcitabine combination exhibited limited anticancer activity in patients with advanced epithelial ovarian and primary peritoneal carcinoma, perhaps because of failure to significantly downregulate the client proteins at clinically achievable exposures.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement
None of the authors have any conflicts of interest to disclose.
Figures

Similar articles
-
Phase I study of 17-allylamino-17 demethoxygeldanamycin, gemcitabine and/or cisplatin in patients with refractory solid tumors.Invest New Drugs. 2011 Jun;29(3):473-80. doi: 10.1007/s10637-009-9381-y. Epub 2010 Jan 15. Invest New Drugs. 2011. PMID: 20082116 Free PMC article. Clinical Trial.
-
Phase II study of gemcitabine and vinorelbine as second- or third-line therapy in patients with primary refractory or platinum-resistant recurrent ovarian and primary peritoneal cancer by the Korean Cancer Study Group (KCSG)_KCSG GY10-10.Gynecol Oncol. 2015 Feb;136(2):212-7. doi: 10.1016/j.ygyno.2014.11.017. Epub 2014 Nov 22. Gynecol Oncol. 2015. PMID: 25462205 Clinical Trial.
-
Phase I and pharmacological study of cytarabine and tanespimycin in relapsed and refractory acute leukemia.Haematologica. 2011 Nov;96(11):1619-26. doi: 10.3324/haematol.2011.049551. Epub 2011 Jul 26. Haematologica. 2011. PMID: 21791475 Free PMC article. Clinical Trial.
-
Bevacizumab combination therapy: a review of its use in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer.BioDrugs. 2013 Aug;27(4):375-92. doi: 10.1007/s40259-013-0043-4. BioDrugs. 2013. PMID: 23728884 Review.
-
Tanespimycin: the opportunities and challenges of targeting heat shock protein 90.Expert Opin Investig Drugs. 2009 Jun;18(6):861-8. doi: 10.1517/13543780902953699. Expert Opin Investig Drugs. 2009. PMID: 19466875 Review.
Cited by
-
Interventions for cough in cancer.Cochrane Database Syst Rev. 2015 May 19;5(5):CD007881. doi: 10.1002/14651858.CD007881.pub3. Cochrane Database Syst Rev. 2015. PMID: 25989380 Free PMC article.
-
Mass Spectrometry-Based Proteomics of Epithelial Ovarian Cancers: A Clinical Perspective.Mol Cell Proteomics. 2023 Jul;22(7):100578. doi: 10.1016/j.mcpro.2023.100578. Epub 2023 May 19. Mol Cell Proteomics. 2023. PMID: 37209814 Free PMC article. Review.
-
Combination Strategies with HSP90 Inhibitors in Cancer Therapy: Mechanisms, Challenges, and Future Perspectives.Pharmaceuticals (Basel). 2025 Jul 22;18(8):1083. doi: 10.3390/ph18081083. Pharmaceuticals (Basel). 2025. PMID: 40872476 Free PMC article. Review.
-
HSP90 inhibition suppresses lipopolysaccharide-induced lung inflammation in vivo.PLoS One. 2015 Jan 23;10(1):e0114975. doi: 10.1371/journal.pone.0114975. eCollection 2015. PLoS One. 2015. PMID: 25615645 Free PMC article.
-
Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies.Cells. 2025 Jul 10;14(14):1057. doi: 10.3390/cells14141057. Cells. 2025. PMID: 40710312 Free PMC article. Review.
References
-
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–28. - PubMed
-
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–72. - PubMed
-
- Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, Moyer JD, DiOrio CI, Barbacci EG, et al. Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogeldanamycin derivatives. J Med Chem. 1995;38:3806–12. - PubMed
-
- Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother Pharmacol. 1998;42:273–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

