Novel therapeutic strategies for metastatic prostate cancer in the post-docetaxel setting
- PMID: 22048000
- PMCID: PMC3233282
- DOI: 10.1634/theoncologist.2010-0412
Novel therapeutic strategies for metastatic prostate cancer in the post-docetaxel setting
Abstract
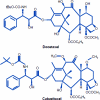
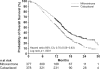
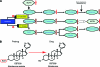
Prostate cancer is the most common noncutaneous cancer and the second leading cause of death from cancer in men in most western countries. Advanced prostate cancer is typically sensitive to androgen-deprivation therapy, but invariably progresses to the castration-resistant state. Most current prostate cancer treatments are based on cytotoxicity directed against tumor cells via androgen-deprivation therapy or chemotherapy. Chemotherapy with docetaxel represents the standard first-line treatment in patients with castration-resistant prostate cancer (CRPC). Following progression after treatment with docetaxel, cabazitaxel (XRP6258)-prednisone treatment leads to a significantly longer overall survival (OS) time than with mitoxantrone-prednisone. Several other novel agents are currently being evaluated, including sipuleucel-T, abiraterone acetate, and MDV3100, as well as the radionuclide alpharadin. The cell-based immunotherapy sipuleucel-T produces longer OS times in chemotherapy-naïve patients, whereas the androgen biosynthesis inhibitor abiraterone acetate results in longer OS times following docetaxel. It is envisioned that these agents will change the standard of care for patients with metastatic CRPC. This review focuses on the clinical development of cabazitaxel and abiraterone acetate.
Conflict of interest statement
Figures
References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- van Vugt HA, Bangma CH, Roobol MJ. Should prostate-specific antigen screening be offered to asymptomatic men? Expert Rev Anticancer Ther. 2010;10:1043–1053. - PubMed
-
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

