Adherens junction protein nectin-4 is the epithelial receptor for measles virus
- PMID: 22048310
- PMCID: PMC3245798
- DOI: 10.1038/nature10639
Adherens junction protein nectin-4 is the epithelial receptor for measles virus
Abstract
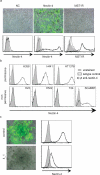
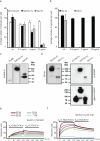
Measles virus is an aerosol-transmitted virus that affects more than 10 million children each year and accounts for approximately 120,000 deaths. Although it was long believed to replicate in the respiratory epithelium before disseminating, it was recently shown to infect initially macrophages and dendritic cells of the airways using signalling lymphocytic activation molecule family member 1 (SLAMF1; also called CD150) as a receptor. These cells then cross the respiratory epithelium and transport the infection to lymphatic organs where measles virus replicates vigorously. How and where the virus crosses back into the airways has remained unknown. On the basis of functional analyses of surface proteins preferentially expressed on virus-permissive human epithelial cell lines, here we identify nectin-4 (ref. 8; also called poliovirus-receptor-like-4 (PVRL4)) as a candidate host exit receptor. This adherens junction protein of the immunoglobulin superfamily interacts with the viral attachment protein with high affinity through its membrane-distal domain. Nectin-4 sustains measles virus entry and non-cytopathic lateral spread in well-differentiated primary human airway epithelial sheets infected basolaterally. It is downregulated in infected epithelial cells, including those of macaque tracheae. Although other viruses use receptors to enter hosts or transit through their epithelial barriers, we suggest that measles virus targets nectin-4 to emerge in the airways. Nectin-4 is a cellular marker of several types of cancer, which has implications for ongoing measles-virus-based clinical trials of oncolysis.
Figures


References
-
- Vaccines: the case of measles. Nature. 2011;473:434–435. doi:10.1038/473434a. - PubMed
-
- Chen SY, et al. Health care-associated measles outbreak in the united states after an importation: challenges and economic impact. J Infect Dis. 2011 doi:10.1093/infdis/jir115. - PubMed
-
- Leonard VH, Hodge G, Reyes-Del Valle J, McChesney MB, Cattaneo R. Measles virus selectively blind to signaling lymphocytic activation molecule (SLAM; CD150) is attenuated and induces strong adaptive immune responses in rhesus monkeys. J Virol. 2010;84:3413–3420. doi:10.1128/JVI.02304-09. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

