Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation
- PMID: 22048766
- PMCID: PMC3375323
- DOI: 10.4049/jimmunol.1102299
Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation
Abstract
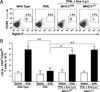
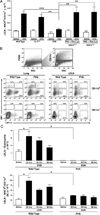
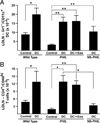
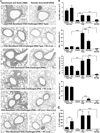
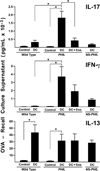
Reports have recently suggested that eosinophils have the potential to modulate allergen-dependent pulmonary immune responses. The studies presented expand these reports demonstrating in the mouse that eosinophils are required for the allergen-dependent Th2 pulmonary immune responses mediated by dendritic cells (DCs) and T lymphocytes. Specifically, the recruitment of peripheral eosinophils to the pulmonary lymphatic compartment(s) was required for the accumulation of myeloid DCs in draining lymph nodes and, in turn, Ag-specific T effector cell production. These effects on DCs and Ag-specific T cells did not require MHC class II expression on eosinophils, suggesting that these granulocytes have an accessory role as opposed to direct T cell stimulation. The data also showed that eosinophils uniquely suppress the DC-mediated production of Th17 and, to smaller degree, Th1 responses. The cumulative effect of these eosinophil-dependent immune mechanisms is to promote the Th2 polarization characteristic of the pulmonary microenvironment after allergen challenge.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures

References
-
- Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol. 2010;126:1081–1091. quiz 1092-1083. - PubMed
-
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Francois-Bernard M. Eosinophilic inflammation in asthma [see comments] New England Journal of Medicine. 1990;323:1033–1039. - PubMed
-
- MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4+ Th2 lymphocyte function. J Immunol. 2001;167:3146–3155. - PubMed
-
- Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

