Cannabinoid receptor 2 is critical for the homing and retention of marginal zone B lineage cells and for efficient T-independent immune responses
- PMID: 22048769
- PMCID: PMC3226756
- DOI: 10.4049/jimmunol.1102195
Cannabinoid receptor 2 is critical for the homing and retention of marginal zone B lineage cells and for efficient T-independent immune responses
Abstract
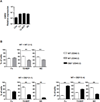
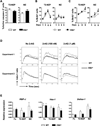
The endocannabinoid system has emerged as an important regulator of immune responses, with the cannabinoid receptor 2 (CB2) and its principle ligand 2-archidonoylglycerol playing a major role. How CB2 regulates B cell functions is not clear, even though they express the highest levels of CB2 among immune cell subsets. In this study, we show that CB2-deficient mice have a significant reduction in the absolute number of marginal zone (MZ) B cells and their immediate precursor, transitional-2 MZ precursor. The loss of MZ lineage cells in CB2(-/-) mice was shown to be B cell intrinsic using bone marrow chimeras and was not due to a developmental or functional defect as determined by B cell phenotype, proliferation, and Ig production. Furthermore, CB2(-/-) B cells were similar to wild type in their apoptosis, cell turnover, and BCR and Notch-2 signaling. We then demonstrated that CB2(-/-) MZ lineage B cells were less efficient at homing to the MZ and that their subsequent retention was also regulated by CB2. CB2(-/-) mice immunized with T-independent Ags produced significantly less Ag-specific IgM. This study demonstrates that CB2 positively regulates T-independent immune responses by controlling the localization and positioning of MZ lineage cells to the MZ.
Figures

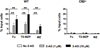



References
-
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. - PubMed
-
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. - PubMed
-
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. - PubMed
-
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

