Several RNase T2 enzymes function in induced tRNA and rRNA turnover in the ciliate Tetrahymena
- PMID: 22049026
- PMCID: PMC3248902
- DOI: 10.1091/mbc.E11-08-0689
Several RNase T2 enzymes function in induced tRNA and rRNA turnover in the ciliate Tetrahymena
Abstract
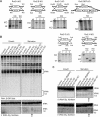
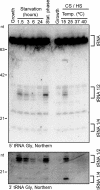
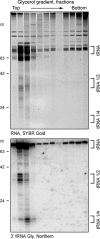
RNase T2 enzymes are produced by a wide range of organisms and have been implicated to function in diverse cellular processes, including stress-induced anticodon loop cleavage of mature tRNAs to generate tRNA halves. Here we describe a family of eight RNase T2 genes (RNT2A-RNT2H) in the ciliate Tetrahymena thermophila. We constructed strains lacking individual or combinations of these RNT2 genes that were viable but had distinct cellular and molecular phenotypes. In strains lacking only one Rnt2 protein or lacking a subfamily of three catalytically inactive Rnt2 proteins, starvation-induced tRNA fragments continued to accumulate, with only a minor change in fragment profile in one strain. We therefore generated strains lacking pairwise combinations of the top three candidates for Rnt2 tRNases. Each of these strains showed a distinct starvation-specific profile of tRNA and rRNA fragment accumulation. These results, the delineation of a broadened range of conditions that induce the accumulation of tRNA halves, and the demonstration of a predominantly ribonucleoprotein-free state of tRNA halves in cell extract suggest that ciliate tRNA halves are degradation intermediates in an autophagy pathway induced by growth arrest that functions to recycle idle protein synthesis machinery.
Figures



References
-
- Acquati F, et al. Tumor and metastasis suppression by the human RNASET2 gene. Int J Oncol. 2005;26:1159–1168. - PubMed
-
- Beau I, Esclatine A, Codogno P. Lost to translation: when autophagy targets mature ribosomes. Trends Cell Biol. 2008;18:311–314. - PubMed
-
- Bendtsen JD, Nielsen H, von HG, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. - PubMed

