Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity
- PMID: 22049070
- PMCID: PMC3321362
- DOI: 10.1126/scitranslmed.3002573
Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity
Abstract
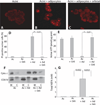
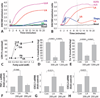
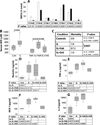
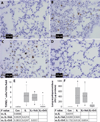
Obesity increases the risk of adverse outcomes during acute critical illnesses such as burns, severe trauma, and acute pancreatitis. Although individuals with more body fat and higher serum cytokines and lipase are more likely to experience problems, the roles that these characteristics play are not clear. We used severe acute pancreatitis as a representative disease to investigate the effects of obesity on local organ function and systemic processes. In obese humans, we found that an increase in the volume of intrapancreatic adipocytes was associated with more extensive pancreatic necrosis during acute pancreatitis and that acute pancreatitis was associated with multisystem organ failure in obese individuals. In vitro studies of pancreatic acinar cells showed that unsaturated fatty acids were proinflammatory, releasing intracellular calcium, inhibiting mitochondrial complexes I and V, and causing necrosis. Saturated fatty acids had no such effects. Inhibition of lipolysis in obese (ob/ob) mice with induced pancreatitis prevented a rise in serum unsaturated fatty acids and prevented renal injury, lung injury, systemic inflammation, hypocalcemia, reduced pancreatic necrosis, and mortality. Thus, therapeutic approaches that target unsaturated fatty acid-mediated lipotoxicity may reduce adverse outcomes in obese patients with critical illnesses such as severe acute pancreatitis.
Figures




Comment in
-
Obesity: Unsaturated fatty acids aggravate acute pancreatitis.Nat Rev Gastroenterol Hepatol. 2011 Dec 6;9(1):5. doi: 10.1038/nrgastro.2011.224. Nat Rev Gastroenterol Hepatol. 2011. PMID: 22143268 No abstract available.
References
-
- Ghanem AM, Sen S, Philp B, Dziewulski P, Shelley OP. Body mass index (BMI) and mortality in patients with severe burns: Is there a “tilt point” at which obesity influences outcome? Burns. 2011;37:208–214. - PubMed
-
- Neville AL, Brown CV, Weng J, Demetriades D, Velmahos GC. Obesity is an independent risk factor of mortality in severely injured blunt trauma patients. Arch. Surg. 2004;139:983–987. - PubMed
-
- Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. Obesity increases risk of organ failure after severe trauma. J. Am. Coll. Surg. 2006;203:539–545. - PubMed
-
- Oliveros H, Villamor E. Obesity and mortality in critically ill adults: A systematic review and meta-analysis. Obesity. 2008;16:515–521. - PubMed
-
- Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: Performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

