ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer
- PMID: 22049316
- PMCID: PMC3204388
- DOI: 10.1158/2159-8290.CD-11-0101
ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer
Abstract
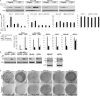
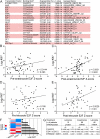
Most estrogen receptor α (ER)-positive breast cancers initially respond to antiestrogens, but many eventually become estrogen-independent and recur. We identified an estrogen-independent role for ER and the CDK4/Rb/E2F transcriptional axis in the hormone-independent growth of breast cancer cells. ER downregulation with fulvestrant or small interfering RNA (siRNA) inhibited estrogen-independent growth. Chromatin immunoprecipitation identified ER genomic binding activity in estrogen-deprived cells and primary breast tumors treated with aromatase inhibitors. Gene expression profiling revealed an estrogen-independent, ER/E2F-directed transcriptional program. An E2F activation gene signature correlated with a lesser response to aromatase inhibitors in patients' tumors. siRNA screening showed that CDK4, an activator of E2F, is required for estrogen-independent cell growth. Long-term estrogen-deprived cells hyperactivate phosphatidylinositol 3-kinase (PI3K) independently of ER/E2F. Fulvestrant combined with the pan-PI3K inhibitor BKM120 induced regression of ER(+) xenografts. These data support further development of ER downregulators and CDK4 inhibitors, and their combination with PI3K inhibitors for treatment of antiestrogen-resistant breast cancers.
Keywords: CDK4; Estrogen receptor; aromatase inhibitor; breast; resistance.
©2011 AACR.
Figures



Comment in
-
ER and PI3K independently modulate endocrine resistance in ER-positive breast cancer.Cancer Discov. 2011 Sep;1(4):287-8. doi: 10.1158/2159-8290.CD-11-0192. Cancer Discov. 2011. PMID: 22096658 Free PMC article.
References
-
- Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. - PubMed
-
- De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–8. - PubMed
-
- Ellis MJ, Tao Y, Young O, White S, Proia AD, Murray J, et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol. 2006;24:3019–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA080195/CA/NCI NIH HHS/United States
- RC1CA145138/CA/NCI NIH HHS/United States
- R01 CA140628/CA/NCI NIH HHS/United States
- K25 CA127349/CA/NCI NIH HHS/United States
- K99 CA142899/CA/NCI NIH HHS/United States
- U24 CA126588/CA/NCI NIH HHS/United States
- F32 CA121900/CA/NCI NIH HHS/United States
- F32CA121900/CA/NCI NIH HHS/United States
- P50CA98131/CA/NCI NIH HHS/United States
- P30CA68485/CA/NCI NIH HHS/United States
- BREAST CANCER NOW RESEARCH CENTRE/BBC_/Breast Cancer Now/United Kingdom
- U24CA126588/CA/NCI NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- K99CA142899/CA/NCI NIH HHS/United States
- K25CA127349/CA/NCI NIH HHS/United States
- RC1 CA145138/CA/NCI NIH HHS/United States
- P50 CA098131/CA/NCI NIH HHS/United States
- R01CA140628/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- T32 CA009592/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

