Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway
- PMID: 22049341
- PMCID: PMC3219098
- DOI: 10.1073/pnas.1109348108
Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway
Abstract
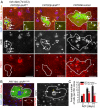
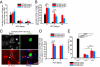
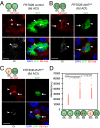
Drosophila adult midgut intestinal stem cells (ISCs) maintain tissue homeostasis by producing progeny that replace dying enterocytes and enteroendocrine cells. ISCs adjust their rates of proliferation in response to enterocyte turnover through a positive feedback loop initiated by secreted enterocyte-derived ligands. However, less is known about whether ISC proliferation is affected by growth of the progeny as they differentiate. Here we show that nutrient deprivation and reduced insulin signaling results in production of growth-delayed enterocytes and prolonged contact between ISCs and newly formed daughters. Premature disruption of cell contact between ISCs and their progeny leads to increased ISC proliferation and rescues proliferation defects in insulin receptor mutants and nutrient-deprived animals. These results suggest that ISCs can indirectly sense changes in nutrient and insulin levels through contact with their daughters and reveal a mechanism that could link physiological changes in tissue growth to stem cell proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
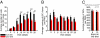

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

