A new Prospero and microRNA-279 pathway restricts CO2 receptor neuron formation
- PMID: 22049409
- PMCID: PMC6623035
- DOI: 10.1523/JNEUROSCI.2592-11.2011
A new Prospero and microRNA-279 pathway restricts CO2 receptor neuron formation
Erratum in
- J Neurosci. 2011 Dec 14;31(50):18627
Abstract
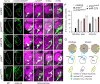
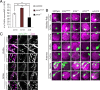
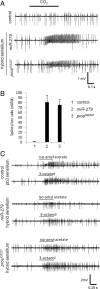
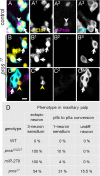
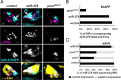
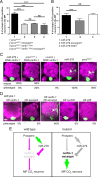
CO(2) sensation represents an interesting example of nervous system and behavioral evolutionary divergence. The underlying molecular mechanisms, however, are not understood. Loss of microRNA-279 in Drosophila melanogaster leads to the formation of a CO(2) sensory system partly similar to the one of mosquitoes. Here, we show that a novel allele of the pleiotropic transcription factor Prospero resembles the miR-279 phenotype. We use a combination of genetics and in vitro and in vivo analysis to demonstrate that Pros participates in the regulation of miR-279 expression, and that reexpression of miR-279 rescues the pros CO(2) neuron phenotype. We identify common target molecules of miR-279 and Pros in bioinformatics analysis, and show that overexpression of the transcription factors Nerfin-1 and Escargot (Esg) is sufficient to induce formation of CO(2) neurons on maxillary palps. Our results suggest that Prospero restricts CO(2) neuron formation indirectly via miR-279 and directly by repressing the shared target molecules, Nerfin-1 and Esg, during olfactory system development. Given the important role of Pros in differentiation of the nervous system, we anticipate that miR-mediated signal tuning represents a powerful method for olfactory sensory system diversification during evolution.
Conflict of interest statement
The authors declare no financial conflicts interest.
Figures




References
-
- Beissbarth T, Speed TP. GOstat: find statistically overrepresented gene ontologies within a group of genes. Bioinformatics. 2004;20:1464–1465. - PubMed
-
- Biryukova I, Asmar J, Abdesselem H, Heitzler P. Drosophila mir-9a regulates wing development via fine-tuning expression of the LIM only factor, dLMO. Dev Biol. 2009;327:487–496. - PubMed
-
- Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BE, van Steensel B, Micklem G, Brand AH. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev Cell. 2006;11:775–789. - PubMed
-
- Cook T, Pichaud F, Sonneville R, Papatsenko D, Desplan C. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev Cell. 2003;4:853–864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
