Arrhythmogenic consequences of myofibroblast-myocyte coupling
- PMID: 22049532
- PMCID: PMC3258652
- DOI: 10.1093/cvr/cvr292
Arrhythmogenic consequences of myofibroblast-myocyte coupling
Abstract
Aims: Fibrosis is known to promote cardiac arrhythmias by disrupting myocardial structure. Given recent evidence that myofibroblasts form gap junctions with myocytes at least in co-cultures, we investigated whether myofibroblast-myocyte coupling can promote arrhythmia triggers, such as early afterdepolarizations (EADs), by directly influencing myocyte electrophysiology.
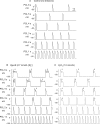
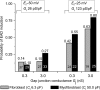
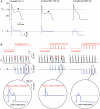
Methods and results: Using the dynamic voltage clamp technique, patch-clamped adult rabbit ventricular myocytes were electrotonically coupled to one or multiple virtual fibroblasts or myofibroblasts programmed with eight combinations of capacitance, membrane resistance, resting membrane potential, and gap junction coupling resistance, spanning physiologically realistic ranges. Myocytes were exposed to oxidative (1 mmol/L H(2)O(2)) or ionic (2.7 mmol/L hypokalaemia) stress to induce bradycardia-dependent EADs. In the absence of myofibroblast-myocyte coupling, EADs developed during slow pacing (6 s), but were completely suppressed by faster pacing (1 s). However, in the presence of myofibroblast-myocyte coupling, EADs could no longer be suppressed by rapid pacing, especially when myofibroblast resting membrane potential was depolarized (-25 mV). Analysis of the myofibroblast-myocyte virtual gap junction currents revealed two components: an early transient-outward I(to)-like current and a late sustained current. Selective elimination of the I(to)-like component prevented EADs, whereas selective elimination of the late component did not.
Conclusion: Coupling of myocytes to myofibroblasts promotes EAD formation as a result of a mismatch in early vs. late repolarization reserve caused by the I(to)-like component of the gap junction current. These cellular and ionic mechanisms may contribute to the pro-arrhythmic risk in fibrotic hearts.
Figures

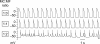

Comment in
-
Myocyte-fibroblast electrical coupling: the basis of a stable relationship?Cardiovasc Res. 2012 Feb 1;93(2):215-7. doi: 10.1093/cvr/cvr338. Epub 2011 Dec 13. Cardiovasc Res. 2012. PMID: 22166713 No abstract available.
References
-
- Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res. 2004;62:415–425. - PubMed
-
- Rook MB, van Ginneken AC, de Jonge B, el Aoumari A, Gros D, Jongsma HJ. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol. 1992;263:C959–C977. - PubMed
-
- Jacquemet V. Pacemaker activity resulting from the coupling with nonexcitable cells. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;74:011908. - PubMed
-
- Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

