Role of ion channels in sepsis-induced atrial tachyarrhythmias in guinea pigs
- PMID: 22050008
- PMCID: PMC3415663
- DOI: 10.1111/j.1476-5381.2011.01769.x
Role of ion channels in sepsis-induced atrial tachyarrhythmias in guinea pigs
Abstract
Background and purpose: Supraventricular tachyarrhythmias, including atrial fibrillation, are occasionally observed in patients suffering from sepsis. Modulation of cardiac ion channel function and expression by sepsis may have a role in the genesis of tachyarrhythmias.
Experimental approach: Sepsis was induced by LPS (i.p.; 300 µg·kg(-1) ) in guinea pigs. Membrane potentials and ionic currents were measured in atrial myocytes isolated from guinea pigs 10 h after LPS, using whole cell patch-clamp methods.
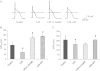
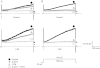
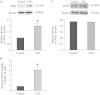
Key results: In atrial cells from LPS-treated animals, action potential duration (APD) was significantly shortened. It was associated with a reduced L-type Ca(2+) current and an increased delayed rectifier K(+) current. These electrophysiological changes were eliminated when N(G) -nitro-l-arginine methyl ester (l-NAME) or S-ethylisothiourea was given together with LPS. In atrial tissues from LPS-treated animals, Ca(2+) channel subunits (Ca(v) 1.2 and Ca(v) 1.3) decreased and delayed rectifier K(+) channel subunits (K(v) 11.1 and K(v) 7.1) increased. However, L-NAME treatment did not substantially reverse such changes in atrial expression in LPS-treated animals, with the exception that K(v) 11.1 subunits returned to control levels. After LPS injection, inducible NOS in atrial tissues was up-regulated, and atrial NO production clearly increased.
Conclusions and implications: In atrial myocytes from guinea pigs with sepsis, APD was significantly shortened. This may reflect nitration of the ion channels which would alter channel functions, rather than changes in atrial expression of the channels. Shortening of APD could serve as one of the mechanisms underlying atrial tachyarrhythmia in sepsis.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


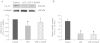

References
-
- Abi-Gerges N, Tavernier B, Mebazaa A, Faivre V, Paqueron X, Payen D, et al. Sequential changes in autonomic regulation of cardiac myocytes after in vivo endotoxin injection in rat. Am J Respir Crit Care Med. 1999;160:1196–1204. - PubMed
-
- Artucio H, Perrier M. Cardiac arrhythmias in critically ill patients: epidemiologic study. Crit Care Med. 1990;18:1383–1388. - PubMed
-
- Bai C-X, Namekata I, Kurokawa J, Tanaka H, Shigenobu K, Furukawa T. Role of nitric oxide in Ca2+ sensitivity of the slowly activating delayed rectifier K+ current in cardiac myocytes. Circ Res. 2005;96:64–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

