Once-daily topical brimonidine tartrate gel 0·5% is a novel treatment for moderate to severe facial erythema of rosacea: results of two multicentre, randomized and vehicle-controlled studies
- PMID: 22050040
- PMCID: PMC3711536
- DOI: 10.1111/j.1365-2133.2011.10716.x
Once-daily topical brimonidine tartrate gel 0·5% is a novel treatment for moderate to severe facial erythema of rosacea: results of two multicentre, randomized and vehicle-controlled studies
Abstract
Background: Erythema of rosacea is thought to result from abnormal cutaneous vasomotor activity. Brimonidine tartrate (BT) is a highly selective α(2) -adrenergic receptor agonist with vasoconstrictive activity.
Objective: To determine the optimal concentration and dose regimen of topical BT gel for the treatment of erythema of rosacea and to evaluate its efficacy and safety.
Methods: In study A, 122 subjects were randomized to receive a single application of BT 0·07%, 0·18%, 0·5% or vehicle. In study B (4-week treatment and 4-week follow-up), 269 subjects were randomized to receive BT 0·5% once daily, BT 0·18% once daily, vehicle once daily, BT 0·18% twice daily or vehicle twice daily. Evaluations included Clinician's Erythema Assessment (CEA), Patient's Self-Assessment (PSA), Chroma Meter measurements and adverse events.
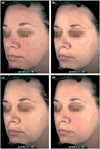
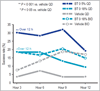
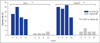
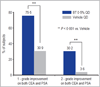
Results: In study A, a single application of topical BT gel reduced facial erythema in a dose-dependent fashion. A significant difference between BT 0·5% and vehicle in Chroma Meter redness value was observed from 30min to 12h after application. In study B, BT 0·5% once daily had a statistically superior success profile (defined as a two-grade improvement on both CEA and PSA over 12h) compared with vehicle once daily on days 1, 15 and 29 (all P<0·001). No tachyphylaxis, rebound of erythema or aggravation of other disease signs (telangiectasia, inflammatory lesions) was observed. All regimens were safe and well tolerated with similarly low incidence of adverse events.
Conclusions: Once-daily BT gel 0·5% is well tolerated and provides significantly greater efficacy than vehicle gel for the treatment of moderate to severe erythema of rosacea.
© 2011 The Authors. BJD © 2011 British Association of Dermatologists.
Conflict of interest statement
Y.L. and M.L. are employees of Galderma R&D.
Figures
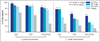


References
-
- Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327–341. - PubMed
-
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584–587. - PubMed
-
- Berg M, Liden S. An epidemiological study of rosacea. Acta Derm Venereol. 1989;69:419–423. - PubMed
-
- Gupta AK, Chaudhry MM. Rosacea and its management: an overview. J Eur Acad Dermatol Venereol. 2005;19:273–285. - PubMed
-
- Wolf JE, Jr, Del Rosso JQ. The CLEAR trial: results of a large community-based study of metronidazole gel in rosacea. Cutis. 2007;79:73–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

