DNA building blocks: keeping control of manufacture
- PMID: 22050358
- PMCID: PMC3267527
- DOI: 10.3109/10409238.2011.630372
DNA building blocks: keeping control of manufacture
Abstract
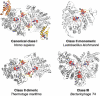
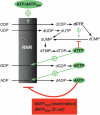
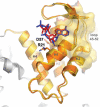
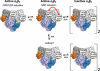
Ribonucleotide reductase (RNR) is the only source for de novo production of the four deoxyribonucleoside triphosphate (dNTP) building blocks needed for DNA synthesis and repair. It is crucial that these dNTP pools are carefully balanced, since mutation rates increase when dNTP levels are either unbalanced or elevated. RNR is the major player in this homeostasis, and with its four different substrates, four different allosteric effectors and two different effector binding sites, it has one of the most sophisticated allosteric regulations known today. In the past few years, the structures of RNRs from several bacteria, yeast and man have been determined in the presence of allosteric effectors and substrates, revealing new information about the mechanisms behind the allosteric regulation. A common theme for all studied RNRs is a flexible loop that mediates modulatory effects from the allosteric specificity site (s-site) to the catalytic site for discrimination between the four substrates. Much less is known about the allosteric activity site (a-site), which functions as an on-off switch for the enzyme's overall activity by binding ATP (activator) or dATP (inhibitor). The two nucleotides induce formation of different enzyme oligomers, and a recent structure of a dATP-inhibited α(6)β(2) complex from yeast suggested how its subunits interacted non-productively. Interestingly, the oligomers formed and the details of their allosteric regulation differ between eukaryotes and Escherichia coli. Nevertheless, these differences serve a common purpose in an essential enzyme whose allosteric regulation might date back to the era when the molecular mechanisms behind the central dogma evolved.
Figures
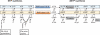
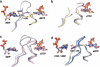

References
-
- Andersson J, Westman M, Hofer A, Sjöberg BM. Allosteric regulation of the class III anaerobic ribonucleotide reductase from bacteriophage T4. J Biol Chem. 2000;275:19443–19448. - PubMed
-
- Aravind L, Wolf YI, Koonin EV. The ATP-cone: an evolutionarily mobile, ATP-binding regulatory domain. J Mol Microbiol Biotechnol. 2000;2:191–194. - PubMed
-
- Averett DR, Lubbers C, Elion GB, Spector T. Ribonucleotide reductase induced by herpes simplex type 1 virus. Characterization of a distinct enzyme. J Biol Chem. 1983;258:9831–9838. - PubMed
-
- Bianchi V, Pontis E, Reichard P. Changes of deoxyribonucleoside triphosphate pools induced by hydroxyurea and their relation to DNA synthesis. J Biol Chem. 1986;261:16037–16042. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
