Cardiomyocytes generated from CPVTD307H patients are arrhythmogenic in response to β-adrenergic stimulation
- PMID: 22050625
- PMCID: PMC3822924
- DOI: 10.1111/j.1582-4934.2011.01476.x
Cardiomyocytes generated from CPVTD307H patients are arrhythmogenic in response to β-adrenergic stimulation
Abstract
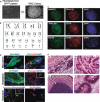
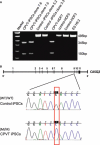
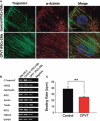
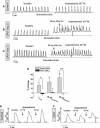
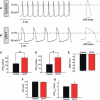
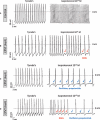
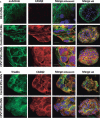
Sudden cardiac death caused by ventricular arrhythmias is a disastrous event, especially when it occurs in young individuals. Among the five major arrhythmogenic disorders occurring in the absence of a structural heart disease is catecholaminergic polymorphic ventricular tachycardia (CPVT), which is a highly lethal form of inherited arrhythmias. Our study focuses on the autosomal recessive form of the disease caused by the missense mutation D307H in the cardiac calsequestrin gene, CASQ2. Because CASQ2 is a key player in excitation contraction coupling, the derangements in intracellular Ca(2+) handling may cause delayed afterdepolarizations (DADs), which constitute the mechanism underlying CPVT. To investigate catecholamine-induced arrhythmias in the CASQ2 mutated cells, we generated for the first time CPVT-derived induced pluripotent stem cells (iPSCs) by reprogramming fibroblasts from skin biopsies of two patients, and demonstrated that the iPSCs carry the CASQ2 mutation. Next, iPSCs were differentiated to cardiomyocytes (iPSCs-CMs), which expressed the mutant CASQ2 protein. The major findings were that the β-adrenergic agonist isoproterenol caused in CPVT iPSCs-CMs (but not in the control cardiomyocytes) DADs, oscillatory arrhythmic prepotentials, after-contractions and diastolic [Ca(2+) ](i) rise. Electron microscopy analysis revealed that compared with control iPSCs-CMs, CPVT iPSCs-CMs displayed a more immature phenotype with less organized myofibrils, enlarged sarcoplasmic reticulum cisternae and reduced number of caveolae. In summary, our results demonstrate that the patient-specific mutated cardiomyocytes can be used to study the electrophysiological mechanisms underlying CPVT.
© 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

References
-
- Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. - PubMed
-
- Viatchenko-Karpinski S, Terentyev D, Gyorke I, et al. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ Res. 2004;94:471–7. - PubMed
-
- di Barletta MR, Viatchenko-Karpinski S, Nori A, et al. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2006;114:1012–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

