BMP4 signaling directs primitive endoderm-derived XEN cells to an extraembryonic visceral endoderm identity
- PMID: 22051107
- PMCID: PMC3246571
- DOI: 10.1016/j.ydbio.2011.10.015
BMP4 signaling directs primitive endoderm-derived XEN cells to an extraembryonic visceral endoderm identity
Abstract
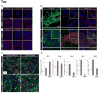
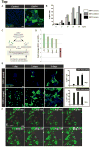
The visceral endoderm (VE) is an epithelial tissue in the early postimplantation mouse embryo that encapsulates the pluripotent epiblast distally and the extraembryonic ectoderm proximally. In addition to facilitating nutrient exchange before the establishment of a circulation, the VE is critical for patterning the epiblast. Since VE is derived from the primitive endoderm (PrE) of the blastocyst, and PrE-derived eXtraembryonic ENdoderm (XEN) cells can be propagated in vitro, XEN cells should provide an important tool for identifying factors that direct VE differentiation. In this study, we demonstrated that BMP4 signaling induces the formation of a polarized epithelium in XEN cells. This morphological transition was reversible, and was associated with the acquisition of a molecular signature comparable to extraembryonic (ex) VE. Resembling exVE which will form the endoderm of the visceral yolk sac, BMP4-treated XEN cells regulated hematopoiesis by stimulating the expansion of primitive erythroid progenitors. We also observed that LIF exerted an antagonistic effect on BMP4-induced XEN cell differentiation, thereby impacting the extrinsic conditions used for the isolation and maintenance of XEN cells in an undifferentiated state. Taken together, our data suggest that XEN cells can be differentiated towards an exVE identity upon BMP4 stimulation and therefore represent a valuable tool for investigating PrE lineage differentiation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

