GnRH receptor activation competes at a low level with growth signaling in stably transfected human breast cell lines
- PMID: 22051164
- PMCID: PMC3227622
- DOI: 10.1186/1471-2407-11-476
GnRH receptor activation competes at a low level with growth signaling in stably transfected human breast cell lines
Abstract
Background: Gonadotrophin releasing hormone (GnRH) analogs lower estrogen levels in pre-menopausal breast cancer patients. GnRH receptor (GnRH-R) activation also directly inhibits the growth of certain cells. The applicability of GnRH anti-proliferation to breast cancer was therefore analyzed.
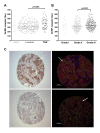
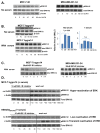
Methods: GnRH-R expression in 298 primary breast cancer samples was measured by quantitative immunofluorescence. Levels of functional GnRH-R in breast-derived cell lines were assessed using 125I-ligand binding and stimulation of 3H-inositol phosphate production. Elevated levels of GnRH-R were stably expressed in cells by transfection. Effects of receptor activation on in vitro cell growth were investigated in comparison with IGF-I and EGF receptor inhibition, and correlated with intracellular signaling using western blotting.
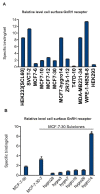
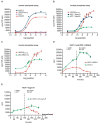
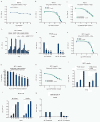
Results: GnRH-R immunoscoring was highest in hormone receptor (triple) negative and grade 3 breast tumors. However prior to transfection, functional endogenous GnRH-R were undetectable in four commonly studied breast cancer cell lines (MCF-7, ZR-75-1, T47D and MDA-MB-231). After transfection with GnRH-R, high levels of cell surface GnRH-R were detected in SVCT and MDA-MB-231 clones while low-moderate levels of GnRH-R occurred in MCF-7 clones and ZR-75-1 clones. MCF-7 sub-clones with high levels of GnRH-R were isolated following hygromycin phosphotransferase transfection. High level cell surface GnRH-R enabled induction of high levels of 3H-inositol phosphate and modest growth-inhibition in SVCT cells. In contrast, growth of MCF-7, ZR-75-1 or MDA-MB-231 clones was unaffected by GnRH-R activation. Cell growth was inhibited by IGF-I or EGF receptor inhibitors. IGF-I receptor inhibitor lowered levels of p-ERK1/2 in MCF-7 clones. Washout of IGF-I receptor inhibitor resulted in transient hyper-elevation of p-ERK1/2, but co-addition of GnRH-R agonist did not alter the dynamics of ERK1/2 re-phosphorylation.
Conclusions: Breast cancers exhibit a range of GnRH-R immunostaining, with higher levels of expression found in triple-negative and grade 3 cancers. However, functional cell surface receptors are rare in cultured cells. Intense GnRH-R signaling in transfected breast cancer cells did not markedly inhibit growth, in contrast to transfected HEK 293 cells indicating the importance of intracellular context. GnRH-R signaling could not counteract IGF-I receptor-tyrosine kinase addiction in MCF-7 cells. These results suggest that combinatorial strategies with growth factor inhibitors will be needed to enhance GnRH anti-proliferative effects in breast cancer.
Figures
References
-
- LHRH-agonists in Early Breast Cancer Overview group. Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, Regan M, Sainsbury R. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007;369:1711–2173. - PubMed
-
- Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rücklinger E, Greil R. ABCSG-12 Trial Investigators. Marth C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. New Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. - DOI - PubMed
-
- Hershkovitz E, Marbach M, Bosin E, Levy J, Roberts CT Jr, LeRoith D, Schally AV, Sharoni Y. Luteinizing hormone-releasing hormone antagonists interfere with autocrine and paracrine growth stimulation of MCF-7 mammary cancer cells by insulin-like growth factors. J Clin Endocrinol Metab. 1993;77:963–968. doi: 10.1210/jc.77.4.963. - DOI - PubMed
-
- Buchholz S, Seitz S, Schally AV, Engel JB, Rick FG, Szalontay L, Hohla F, Krishan A, Papadia A, Gaiser T, Brockhoff G, Ortmann O, Diedrich K, Köster F. Triple-negative breast cancers express receptors for luteinizing hormone-releasing hormone (LHRH) and respond to LHRH antagonist cetrorelix with growth inhibition. Int J Oncol. 2009;35:789–796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

