Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP)
- PMID: 22051941
- PMCID: PMC3270297
- DOI: 10.1038/oby.2011.330
Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP)
Abstract
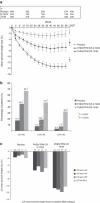
A 56-week randomized controlled trial was conducted to evaluate safety and efficacy of a controlled-release combination of phentermine and topiramate (PHEN/TPM CR) for weight loss (WL) and metabolic improvements. Men and women with class II and III obesity (BMI ≥ 35 kg/m(2)) were randomized to placebo, PHEN/TPM CR 3.75/23 mg, or PHEN/TPM CR 15/92 mg, added to a reduced-energy diet. Primary end points were percent WL and proportions of patients achieving 5% WL. Secondary end points included waist circumference (WC), systolic and diastolic blood pressure (BP), fasting glucose, and lipid measures. In the primary analysis (randomized patients with at least one postbaseline weight measurement who took at least one dose of assigned drug or placebo), patients in the placebo, 3.75/23, and 15/92 groups lost 1.6%, 5.1%, and 10.9% of baseline body weight (BW), respectively, at 56 weeks (P < 0.0001). In categorical analysis, 17.3% of placebo patients, 44.9% of 3.75/23 patients, and 66.7% of 15/92 patients, lost at least 5% of baseline BW at 56 weeks (P < 0.0001). The 15/92 group had significantly greater changes relative to placebo for WC, systolic and diastolic BP, fasting glucose, triglycerides, total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL). The most common adverse events were paresthesia, dry mouth, constipation, dysgeusia, and insomnia. Dropout rate from the study was 47.1% for placebo patients, 39.0% for 3.75/23 patients, and 33.6% of 15/92 patients. PHEN/TPM CR demonstrated dose-dependent effects on weight and metabolic variables in the direction expected to be beneficial with no evidence of serious adverse events induced by treatment.
Figures

References
-
- Allison DB, Downey M, Atkinson RL.et al. Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of the Obesity Society Obesity (Silver Spring) 2008161161–1177. - PubMed
-
- Pi-Sunyer FX. A review of long-term studies evaluating the efficacy of weight loss in ameliorating disorders associated with obesity. Clin Ther. 1996;18:1006–35; discussion 1005. - PubMed
-
- Franz MJ, VanWormer JJ, Crain AL.et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up J Am Diet Assoc 20071071755–1767. - PubMed
-
- Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M, OBES-002 Study Group A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28:1399–1410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

