Resveratrol causes cell cycle arrest, decreased collagen synthesis, and apoptosis in rat intestinal smooth muscle cells
- PMID: 22052016
- PMCID: PMC3287405
- DOI: 10.1152/ajpgi.00083.2011
Resveratrol causes cell cycle arrest, decreased collagen synthesis, and apoptosis in rat intestinal smooth muscle cells
Abstract
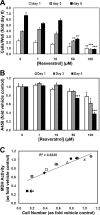
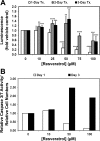
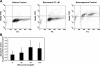
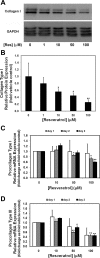
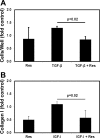
One of the most difficult and treatment-resistant complications of Crohn's disease is the development of fibrotic intestinal strictures due to mesenchymal cell hyperplasia and collagen deposition. Resveratrol, a phytoalexin found in berries, peanuts, grapes, and red wine, has been shown to inhibit fibrosis in vasculature, heart, lung, kidney, liver, and esophagus in animal models. Resveratrol has also been shown to inhibit oxidation, inflammation, and cell proliferation and to decrease collagen synthesis in several cell types or animal models. The aim of this study was to determine whether resveratrol has antifibrotic effects on intestinal smooth muscle cells. Responses to resveratrol by cultured smooth muscle cells isolated from colons of untreated Lewis rats were examined; this rat strain is used in a model of Crohn's disease with prominent intestinal fibrosis. A relative decrease in cell numbers following treatment with 50 and 100 μM resveratrol was evident at 24 h (P ≤ 0.005). This effect was largely due to cell cycle arrest, with an increase in the percent of cells in S phase from 8 to 25-35% (P < 0.05). Cell viability was unchanged until 2-3 days of treatment when there was a 1.2- to 5.0-fold increase in the percent of apoptotic cells, depending on the assay (P < 0.05). Expression of collagen type I protein was decreased following treatment with resveratrol for 24 h (to 44 and 25% of control levels with 50 and 100 μM resveratrol, respectively; P < 0.05). Expression of procollagen types I and III mRNA was also decreased with resveratrol treatment. Resveratrol (50 μM) diminished the proliferative response to TGF-β₁ (P = 0.02) as well as IGF-I-stimulated collagen production (P = 0.02). Thus resveratrol decreases intestinal smooth muscle cell numbers through its effects on cell cycle arrest and apoptosis and also decreases collagen synthesis by the cells. These effects could be useful in preventing the smooth muscle cell hyperplasia and collagen deposition that characterize stricture formation in Crohn's disease.
Figures

References
-
- Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha J-F, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res 53: S7–S15, 2009 - PubMed
-
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol 12: 141–179, 1994 - PubMed
-
- Banerejee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-κB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res 62: 4945–4954, 2002 - PubMed
-
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506, 2006 - PubMed
-
- Boocock DJ, Faust GES, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev 16: 1246–1252, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

