Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta
- PMID: 22052668
- PMCID: PMC3725821
- DOI: 10.1002/humu.21647
Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta
Abstract
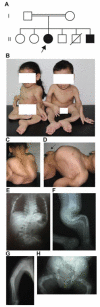
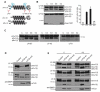
Herein, we have studied a consanguineous Egyptian family with two children diagnosed with severe autosomal recessive osteogenesis imperfecta (AR-OI) and a large umbilical hernia. Homozygosity mapping in this family showed lack of linkage to any of the previously known AR-OI genes, but revealed a 10.27 MB homozygous region on chromosome 8p in the two affected sibs, which comprised the procollagen I C-terminal propeptide (PICP) endopeptidase gene BMP1. Mutation analysis identified both patients with a Phe249Leu homozygous missense change within the BMP1 protease domain involving a residue, which is conserved in all members of the astacin group of metalloproteases. Type I procollagen analysis in supernatants from cultured fibroblasts demonstrated abnormal PICP processing in patient-derived cells consistent with the mutation causing decreased BMP1 function. This was further confirmed by overexpressing wild type and mutant BMP1 longer isoform (mammalian Tolloid protein [mTLD]) in NIH3T3 fibroblasts and human primary fibroblasts. While overproduction of normal mTLD resulted in a large proportion of proα1(I) in the culture media being C-terminally processed, proα1(I) cleavage was not enhanced by an excess of the mutant protein, proving that the Phe249Leu mutation leads to a BMP1/mTLD protein with deficient PICP proteolytic activity. We conclude that BMP1 is an additional gene mutated in AR-OI.
© 2011 Wiley Periodicals, Inc.
Figures

References
-
- Alanay Y, Avaygan H, Camacho N, Utine GE, Boduroglu K, Aktas D, Alikasifoglu M, Tuncbilek E, Orhan D, Bakar FT, Zabel B, Superti-Furga A, Bruckner-Tuderman L, Curry CJ, Pyott S, Byers PH, Eyre DR, Baldridge D, Lee B, Merrill AE, Davis EC, Cohn DH, Akarsu N, Krakow D. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86:551–559. - PMC - PubMed
-
- Barnes AM, Chang W, Morello R, Cabral WA, Weis M, Eyre DR, Leikin S, Makareeva E, Kuznetsova N, Uveges TE, Ashok A, Flor AW, Mulvihill JJ, Wilson PL, Sundaram UT, Lee B, Marini JC. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006;355:2757–2764. - PMC - PubMed
-
- Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, Giunta C, Bergmann C, Rohrbach M, Koerber F, Zimmermann K, de Vries P, Wirth B, Schoenau E, Wollnik B, Veltman JA, Hoischen A, Netzer C. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2011;88:362–371. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

