Boosting heterosubtypic neutralization antibodies in recipients of 2009 pandemic H1N1 influenza vaccine
- PMID: 22052887
- PMCID: PMC3243653
- DOI: 10.1093/cid/cir753
Boosting heterosubtypic neutralization antibodies in recipients of 2009 pandemic H1N1 influenza vaccine
Abstract
Background: A mass vaccination has been implemented to prevent the spread of 2009 pandemic influenza virus in China. Highly limited information is available on whether this vaccine induces cross-reactive neutralization antibodies against other subtypes of influenza viruses.
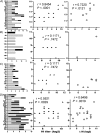
Methods: We employed pseudovirus-based assays to analyze heterosubtypic neutralization responses in serum samples of 23 recipients of 2009 pandemic influenza vaccine.
Results: One dose of pandemic vaccine not only stimulated good neutralization antibodies against cognate influenza virus 2009 influenza A (H1N1), but also raised broad cross-reactive neutralization activities against seasonal H3N2 and highly pathogenic avian influenza virus H5N1 and lesser to H2N2. The cross-reactive neutralization activities were completely abolished after the removal of immunoglobin G (IgG). In contrast, H1N1 vaccination alone in influenza-naive mice elicited only vigorous homologous neutralizing activities but not cross-reactive neutralization activities.
Conclusions: Our data suggest that the cross-reactive neutralization epitopes do exist in this vaccine and could elicit significant cross-reactive neutralizing IgG antibodies in the presence of preexisting responses. The exposure to H1N1 vaccine is likely to modify the hierarchical order of preexisting immune responses to influenza viruses. These findings provide insights into the evolution of human immunity to influenza viruses after experiencing multiple influenza virus infections and vaccinations.
Figures


References
-
- Smith GJ, Vijaykrishna D, Bahl J, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–5. - PubMed
-
- Zhu FC, Wang H, Fang HH, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–23. - PubMed
-
- Wu J, Xu F, Lu L, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med. 2010;363:2416–23. - PubMed
-
- Liang XF, Wang HQ, Wang JZ, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

