Divalent cation transport by vesicular nucleotide transporter
- PMID: 22052906
- PMCID: PMC3234814
- DOI: 10.1074/jbc.M111.277269
Divalent cation transport by vesicular nucleotide transporter
Abstract
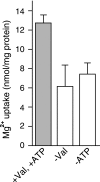
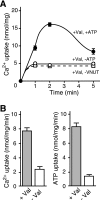
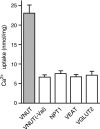
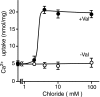
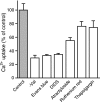
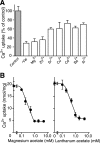
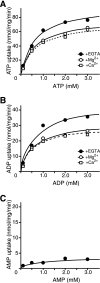
The vesicular nucleotide transporter (VNUT) is a secretory vesicle protein that is responsible for the vesicular storage and subsequent exocytosis of ATP (Sawada, K., Echigo, N., Juge, N., Miyaji, T., Otsuka, M., Omote, H., and Moriyama, Y. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5683-5686). Because VNUT actively transports ATP in a membrane potential (Δψ)-dependent manner irrespective of divalent cations such as Mg(2+) and Ca(2+), VNUT recognizes free ATP as a transport substrate. However, whether or not VNUT transports chelating complexes with divalent cations remains unknown. Here, we show that proteoliposomes containing purified VNUT actively took up Mg(2+) when ATP was present, as detected by atomic absorption spectroscopy. The VNUT-containing proteoliposomes also took up radioactive Ca(2+) upon imposing Δψ (positive-inside) but not ΔpH. The Δψ-driven Ca(2+) uptake required ATP and a millimolar concentration of Cl(-), which was inhibited by Evans blue, a specific inhibitor of SLC17-type transporters. VNUT in which Arg-119 was specifically mutated to alanine, the counterpart of the essential amino acid residue of the SLC17 family, lost the ability to take up both ATP and Ca(2+). Ca(2+) uptake was also inhibited in the presence of various divalent cations such as Mg(2+). Kinetic analysis indicated that Ca(2+) or Mg(2+) did not affect the apparent affinity for ATP. RNAi of the VNUT gene in PC12 cells decreased the vesicular Mg(2+) concentration to 67.7%. These results indicate that VNUT transports both nucleotides and divalent cations probably as chelating complexes and suggest that VNUT functions as a divalent cation importer in secretory vesicles under physiological conditions.
Figures
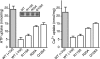
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

