Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses
- PMID: 22052977
- PMCID: PMC3471535
- DOI: 10.1126/science.1211485
Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses
Abstract
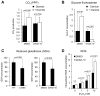
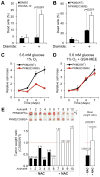
Control of intracellular reactive oxygen species (ROS) concentrations is critical for cancer cell survival. We show that, in human lung cancer cells, acute increases in intracellular concentrations of ROS caused inhibition of the glycolytic enzyme pyruvate kinase M2 (PKM2) through oxidation of Cys(358). This inhibition of PKM2 is required to divert glucose flux into the pentose phosphate pathway and thereby generate sufficient reducing potential for detoxification of ROS. Lung cancer cells in which endogenous PKM2 was replaced with the Cys(358) to Ser(358) oxidation-resistant mutant exhibited increased sensitivity to oxidative stress and impaired tumor formation in a xenograft model. Besides promoting metabolic changes required for proliferation, the regulatory properties of PKM2 may confer an additional advantage to cancer cells by allowing them to withstand oxidative stress.
Figures


Comment in
-
Cell biology. Warburg effect and redox balance.Science. 2011 Dec 2;334(6060):1219-20. doi: 10.1126/science.1215637. Science. 2011. PMID: 22144609 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

