Determinants of microvascular network topologies in implanted neovasculatures
- PMID: 22053070
- PMCID: PMC3256738
- DOI: 10.1161/ATVBAHA.111.238725
Determinants of microvascular network topologies in implanted neovasculatures
Abstract
Objective: During neovascularization, the end result is a new functional microcirculation composed of a network of mature microvessels with specific topologies. Although much is known concerning the mechanisms underlying the initiation of angiogenesis, it remains unclear how the final architecture of microcirculatory beds is regulated. To begin to address this, we determined the impact of angiogenic neovessel prepatterning on the final microvascular network topology using a model of implant neovascularization.
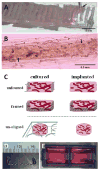
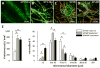
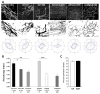
Methods and results: We used 3D direct-write bioprinting or physical constraints in a manner permitting postangiogenesis vascular remodeling and adaptation to pattern angiogenic microvascular precursors (neovessels formed from isolated microvessel segments) in 3D collagen gels before implantation and subsequent network formation. Neovasculatures prepatterned into parallel arrays formed functional networks after 4 weeks postimplantation but lost the prepatterned architecture. However, maintenance of uniaxial physical constraints during postangiogenesis remodeling of the implanted neovasculatures produced networks with aligned microvessels, as well as an altered proportional distribution of arterioles, capillaries, and venules.
Conclusions: Here we show that network topology resulting from implanted microvessel precursors is independent from prepatterning of precursors but can be influenced by a patterning stimulus involving tissue deformation during postangiogenesis remodeling and maturation.
Conflict of interest statement
Figures



References
-
- Pittman RN. Oxygen transport and exchange in the microcirculation. Microcirculation. 2005;12:59–70. - PubMed
-
- Pries AR, Secomb TW. Control of blood vessel structure: insights from theoretical models. Am J Physiol Heart Circ Physiol. 2005;288:H1010–H1015. - PubMed
-
- Hoying JB, Williams SK. Building blood vessels. In: Aird WC, editor. Endothelial Biomedicine. Cambridge: Cambridge University Press; 2007.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

